Continuous Blood Donation for the Development of the Microvisk PT/INR
Microvisk Technologies Ltd designs and develops test systems for monitoring the effectiveness of Warfarin and Warfarin-like medications.
Warfarin and Warfarin-like medications are used to reduce the risk of stroke in people with an irregular heart rhythm (Atrial Fibrillation) or with mechanical heart valve replacements, as well as reducing the risk of recurrent Venous Thromboembolism.
Warfarin dosing needs to be individualized. In order to correctly dose an individual in order to optimize the effectiveness of warfarin, and minimize the risk of bleeding, close monitoring of the degree of anticoagulation is required through regular blood testing. This test is known as PT/INR (Prothrombin Time/International Normalised Ratio). Simply, it is the measure of the amount of time (in seconds) it takes for your blood to clot.
Microvisk Technologies Ltd are developing new anticoagulant test strips for patients on warfarin or warfarin like medications. The blood collected as part of this study will be tested upon Microvisk's second generation Prothrombin/International Normalized Ratio (PT/INR) Test System. The donated blood will be used on ongoing development, validation, verification, and calibration projects. This study is being performed in partnership with the Oxford University Hospital (OUH) and Local Clinical Research Networks (LCRN) Thames Valley Core team, part of National Institute for Health and Care Research (NIHR).
A Prospective, Single-Center Study, in Healthy Volunteers to Establish a PT/INR Reference Interval for the Microvisk INR Test System
This is a prospective, single-center study in healthy volunteers to establish a PT/INR reference interval for the Microvisk International Normalized Ratio (INR) Test System. The primary objective of this study is to establish the reference interval for the measurement of prothrombin time (PT/INR) using the Microvisk INR Test System. The second objective is to evaluate the safety of the device.
Blood Donation for the Development and Optimisation of the Second Generation Microvisk International Normalized Ratio (INR) Testing System for the Measurement of Prothrombin Time (PT)/INR in Patients on Warfarin Therapy.
This study is to further develop and optimise the design and manufacturing process of a handheld device to monitor and manage Warfarin (blood thinning anticoagulation drug) therapy. The device comprises of a handheld instrument and a disposable test strip and reports how blood coagulation is working in terms of standardised units called International Normalised Ratio (INR). A single drop of fresh whole blood and plasma will be added to the strip and the INR result displayed on the instrument. Blood samples are to be collected from patients attending a hospital based INR clinic who are on Warfarin Therapy. The samples are to be used in a series of experiments in the laboratory to test the Microvisk POC INR Monitors accuracy, precision, stability and robustness.
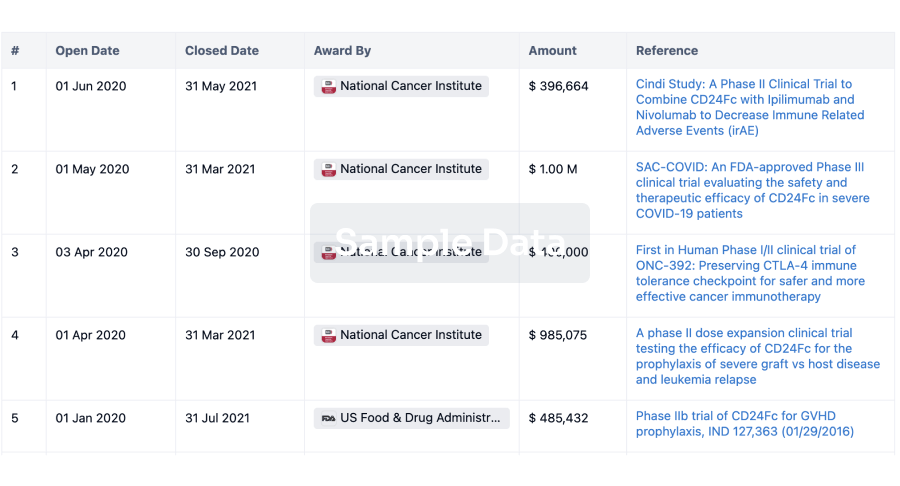
100 Clinical Results associated with Microvisk Ltd.
0 Patents (Medical) associated with Microvisk Ltd.
100 Deals associated with Microvisk Ltd.
100 Translational Medicine associated with Microvisk Ltd.