Request Demo
Last update 09 Sep 2025

PTC Therapeutics, Inc.
Last update 09 Sep 2025
Overview
Tags
Nervous System Diseases
Other Diseases
Congenital Disorders
Small molecule drug
AAV based gene therapy
ASO
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Disease Domain | Count |
|---|---|
| Nervous System Diseases | 17 |
| Endocrinology and Metabolic Disease | 6 |
| Neoplasms | 2 |
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 12 |
| AAV based gene therapy | 4 |
| Unknown | 2 |
| ASO | 1 |
| Gene therapy | 1 |
Related
21
Drugs associated with PTC Therapeutics, Inc.Target |
Mechanism eNOS modulators |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. European Union [+3] |
First Approval Date19 Jun 2025 |
Target |
Mechanism Dystroglycan stimulants |
Active Org. |
Originator Org. |
Active Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. European Union [+3] |
First Approval Date31 Jul 2014 |
95
Clinical Trials associated with PTC Therapeutics, Inc.CTIS2023-506238-61-00
A Phase 3, Randomized, Crossover, Open-Label, Active-Controlled Study of Sepiapterin versus Sapropterin in Participants With Phenylketonuria =2 years of Age - PTC923-PKU-301
Start Date14 May 2024 |
Sponsor / Collaborator |
NCT06302348
A Phase 3b Open-Label Study of Long-Term Neurocognitive Outcomes in Children With Phenylketonuria Treated With Sepiapterin
The main purpose of this trial is to evaluate the long-term efficacy of sepiapterin on preserving neurocognitive functioning in children with PKU when treatment is initiated in early childhood.
Start Date04 Mar 2024 |
Sponsor / Collaborator |
ISRCTN79102999
A Phase III, randomized, crossover, open-label, active-controlled study of sepiapterin versus sapropterin in participants with phenylketonuria =2 years of age
Start Date31 Jan 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with PTC Therapeutics, Inc.
Login to view more data
0 Patents (Medical) associated with PTC Therapeutics, Inc.
Login to view more data
106
Literatures (Medical) associated with PTC Therapeutics, Inc.01 Jan 2025·Journal of Neuromuscular Diseases
In response to Gulcin Akinci’s and Haluk Topaloglu's letter regarding our article “Predictors of loss of ambulation in Duchenne muscular dystrophy: A systematic review and meta-analysis”
Letter
Author: Landfeldt, E ; Werner, C ; Kirschner, J ; Alemán, A ; Lochmüller, H ; Tomazos, I ; Ferizovic, N ; Zhang, R ; Abner, S
01 Jan 2025·Genetic Testing and Molecular Biomarkers
REVEAL-CP: Selective Screening of Pediatric Patients for Aromatic L -Amino Acid Decarboxylase Deficiency with a Guthrie Card and In Silico Structural Modeling of One Index Case
Article
Author: Kuster, Alice ; Leuzzi, Vincenzo ; Liu, Emelline ; Gowda, Vasantha ; Amin, Sam ; Perduca, Massimiliano ; Strehle, Eugen-Matthias ; Bertoldi, Mariarita ; Battini, Roberta ; Johnson, Shelley ; Fox, Emily ; Werner, Christian ; Lupo, Paul
Background: The main objective of this prospective, multicenter study (REVEAL-CP) was to test children with cerebral palsy-like signs and symptoms for raised 3-O-methyldopa (3-OMD) blood levels, a biomarker for aromatic L-amino acid decarboxylase deficiency (AADCd). A secondary objective was to characterize the molecular basis for the defective aromatic L-amino acid decarboxylase (AADC) gene product. Methods: Patients were identified in pediatric secondary and tertiary care hospitals through database searches and personal communication. 3-OMD concentrations from Guthrie card tests were determined using liquid chromatography/mass spectrometry. If 3-OMD was raised, cerebrospinal fluid analysis and dopa decarboxylase (DDC) gene sequencing were performed. An in-silico mutagenesis analysis was carried out to model altered AADC enzymes. Results: In total, 166 patients were enrolled in this study. The median age was 8 years. Sixty-six patients (39.8%) had a diagnosis of cerebral palsy, with the most common type being "mixed" (n = 42; 25.3%). One patient (0.6%), an 11-month-old boy from Italy, was diagnosed with AADCd caused by a homozygous, pathogenic DDC variant (c.749C>T; p.Ser250Phe). Three-dimensional modeling of the Ser250Phe AADC enzyme variant revealed its destabilization. Conclusions: A Guthrie card test for 3-OMD is a recognized screening technique for AADCd. If universal newborn screening for this metabolic disease is not available, children with signs and symptoms of a movement disorder should be investigated for AADCd.
01 Oct 2024·MOLECULAR THERAPY
Preclinical studies of gene replacement therapy for CDKL5 deficiency disorder
Article
Author: Narasimhan, Jana ; Wu, Michael C ; Kim, Min Jung ; Cao, Liangxian ; Wu, Zhijian ; Lee, Jeanee ; Southgate, Christopher ; Pear, Lisset ; Fotouh, Eman ; Pick, Joseph ; Voronin, Gregory ; DeMarco, Steven ; Sheedy, Josephine ; Jung, Stephen ; Peters, Melinda ; Saadipour, Khalil ; Varganov, Yakov ; Weetall, Marla ; Yalamanchili, Padmaja ; Sinha, Supriya ; Welch, Ellen M ; Lipari, Philip ; Gittens, Jamila ; Ray, Balmiki ; Mollin, Anna
Cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) is a rare neurodevelopmental disorder caused by a mutation in the X-linked CDKL5 gene. CDKL5 is a serine/threonine kinase that is critical for axon outgrowth and dendritic morphogenesis as well as synapse formation, maturation, and maintenance. This disorder is characterized by early-onset epilepsy, hypotonia, and failure to reach cognitive and motor developmental milestones. Because the disease is monogenic, delivery of the CDKL5 gene to the brain of patients should provide clinical benefit. To this end, we designed a gene therapy vector, adeno-associated virus (AAV)9.Syn.hCDKL5, in which human CDKL5 gene expression is driven by the synapsin promoter. In biodistribution studies conducted in mice, intracerebroventricular (i.c.v.) injection resulted in broader, more optimal biodistribution than did intra-cisterna magna (i.c.m.) delivery. AAV9.Syn.hCDKL5 treatment increased phosphorylation of EB2, a bona fide CDKL5 substrate, demonstrating biological activity in vivo. Our data provide proof of concept that i.c.v. delivery of AAV9.Syn.hCDKL5 to neonatal male Cdkl5 knockout mice reduces pathology and reduces aberrant behavior. Functional improvements were seen at doses of 3e11 to 5e11 vector genomes/g brain, which resulted in transfection of ≥50% of the neurons. Functional improvements were not seen at lower doses, suggesting a requirement for broad distribution for efficacy.
456
News (Medical) associated with PTC Therapeutics, Inc.05 Sep 2025
Rising prevalence of genetic disorders, supportive regulatory frameworks, and advancements in gene and RNA-based therapies are fueling market expansion.
WILMINGTON, DE, UNITED STATES, September 5, 2025 /
EINPresswire.com
/ -- The global
nucleic acid therapeutics market
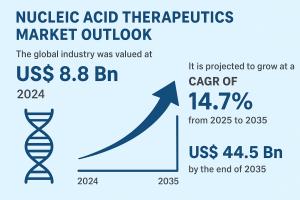
is entering a phase of accelerated growth, driven by strong innovation pipelines and favorable policy support. Valued at US$ 8.8 Bn in 2024, the market is projected to grow at a robust CAGR of 14.7% between 2025 and 2035, reaching US$ 44.5 Bn by 2035. Growing awareness of genetic diseases, widespread clinical trials, and rapid progress in RNA-based platforms are reshaping the future of precision medicine.
Market Introduction
Nucleic acid therapeutics represent one of the most transformative innovations in modern biotechnology. These therapies leverage engineered DNA and RNA molecules to correct or silence defective genes, introduce beneficial genetic material, or modulate protein expression. Core modalities include gene therapies, antisense oligonucleotides (ASOs), RNA interference (RNAi), aptamers, and messenger RNA (mRNA)-based therapeutics.
Their therapeutic applications extend across neuromuscular disorders, oncology, viral infections, ophthalmological diseases, metabolic syndromes, and autoimmune conditions, providing high specificity, targeted action, and the potential for one-time curative treatments. Unlike traditional pharmaceuticals, nucleic acid therapeutics address the root cause of diseases at the genetic level, minimizing systemic toxicity and enabling personalized medicine.
Dive Deeper into Data: Get Your In-Depth Sample Now -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86333
Analyst Viewpoint
Analysts at Transparency Market Research note that the nucleic acid therapeutics market is on the cusp of becoming a mainstream segment in the biopharmaceutical industry.
Key growth catalysts include:
Rising prevalence of rare and inherited disorders such as Duchenne muscular dystrophy, cystic fibrosis, and sickle cell anemia, which remain underserved by conventional treatments.
Regulatory acceleration through FDA Fast Track, Breakthrough Therapy, and EMA Priority Medicines (PRIME) designations, which shorten development timelines for critical therapies.
Challenges remain in terms of manufacturing scalability, delivery mechanisms, and cost-effectiveness, particularly for therapies requiring complex viral or lipid nanoparticle vectors. However, the success of mRNA vaccines during the COVID-19 pandemic has proven the scalability and adaptability of RNA-based platforms, catalyzing confidence among investors and policymakers. The increasing number of clinical trials, coupled with strategic collaborations, points to a market that is set to transform global healthcare by 2035.
Key Market Drivers
1. Rising Prevalence of Genetic Disorders
Genetic diseases affect millions worldwide, with a growing number being diagnosed through advancements in genomic sequencing and prenatal screening. This improved detection rate is expanding the eligible patient pool for nucleic acid therapeutics. As these therapies directly correct or silence defective genes, their adoption is expected to increase significantly in the coming years.
2. Regulatory Approvals and Expedited Pathways
Regulatory agencies such as the U.S. FDA and the European Medicines Agency (EMA) are creating streamlined approval frameworks for nucleic acid therapeutics, particularly in the area of rare and orphan diseases. Expedited review processes reduce time-to-market, encouraging pharmaceutical and biotech companies to increase investment in clinical development.
3. Advancements in RNA-based and Gene Therapies
Breakthroughs in antisense oligonucleotides, siRNA, and mRNA platforms are enabling therapies that target diseases previously considered untreatable. The integration of artificial intelligence, nanotechnology-driven delivery systems, and improved chemical modifications has enhanced drug stability, precision targeting, and reduced off-target effects, making RNA-based treatments commercially viable.
Segment Analysis
By Therapy Type
Antisense Oligonucleotides (ASOs): Largest segment due to their efficacy in modulating gene expression; widely used in neuromuscular and rare genetic disorders.
Small Interfering RNA (siRNA): Emerging as a strong segment with applications in oncology and metabolic diseases.
Gene Therapies: High growth potential owing to curative benefits in monogenic disorders.
Aptamers: Gaining momentum in targeted oncology treatments.
Others (including mRNA therapies): Strong future growth expected, particularly post-COVID-19 success.
By Delivery Method
Viral Vector-based Systems: Widely used but face challenges in immunogenicity and manufacturing scalability.
Non-viral Delivery Systems: Lipid nanoparticles and polymer-based carriers showing promising safety and efficiency.
By Route of Administration
Intravenous: Dominant due to systemic distribution.
Subcutaneous: Preferred for ease of administration and chronic therapies.
Others (Intrathecal, etc.): Specialized routes for targeted disease conditions.
By Therapeutic Area
Neuromuscular Disorders (DMD, SMA)
Metabolic Disorders
Cardiovascular Disorders
Ophthalmological Disorders
Oncological Disorders
Others (Infectious, Autoimmune, etc.)
By End-user
Hospitals (largest share due to advanced care infrastructure)
Academic and Research Institutes (driving clinical trials and innovation)
Specialty Centers (focusing on genetic and rare disorders)
Regional Insights
North America: Leads the global market due to strong biotech infrastructure, presence of leading companies, and early adoption of innovative therapies. Favorable reimbursement policies and active regulatory support enhance growth prospects.
Europe: Rapid adoption supported by EMA’s proactive stance on genetic therapies. Strong research ecosystems in Germany, the UK, and France are advancing innovation.
Asia Pacific: Poised for fastest growth with rising healthcare investments, supportive government initiatives, and a growing patient pool in China, India, and Japan.
Latin America & Middle East & Africa: Emerging regions where adoption is gradually increasing, though affordability and access remain challenges. Partnerships with global biotech firms are expected to enhance growth.
Key Players
Novartis AG
Pfizer, Inc.
Sanofi
Novo Nordisk A/S
AstraZeneca plc
Alnylam Pharmaceuticals, Inc.
Amgen Inc.
Sarepta Therapeutics, Inc.
Bluebird Bio, Inc.
CSL Behring LLC
Ferring Pharmaceuticals Inc.
Krystal Biotech, Inc.
PTC Therapeutics, Inc.
Jazz Pharmaceuticals plc
Astellas Pharma Inc.
Recent Developments
Novartis (Nov 2024): Acquired Kate Therapeutics, expanding its AAV-based gene therapy pipeline for neuromuscular diseases in a deal valued up to US$ 1.1 Bn.
Sarepta Therapeutics (Nov 2024): Entered into a licensing agreement with Arrowhead Pharmaceuticals for siRNA programs in muscle and rare pulmonary disorders, valued at US$ 825 Mn.
Market Trends
Accelerating investments in RNA-based and gene-editing platforms.
Rising demand for personalized and precision medicine approaches.
Continuous technological innovations in viral and non-viral delivery platforms.
Increasing collaborations between pharma companies, biotech firms, and academic institutions to fast-track clinical pipelines.
Future Outlook
The nucleic acid therapeutics market is expected to remain one of the fastest-growing sectors within biotechnology and pharmaceuticals through 2035.
Factors such as increasing prevalence of genetic disorders, regulatory acceleration, advancements in RNA and gene therapy platforms, and strong demand for personalized medicine will drive market expansion. With a projected CAGR of 14.7%, the sector is set to deliver groundbreaking treatments that could redefine modern healthcare.
Why Buy This Report?
Reliable market size forecasts and CAGR projections through 2035
In-depth assessment of market drivers, restraints, and opportunities
Comprehensive segmentation by therapy type, delivery method, therapeutic area, and region
Competitive landscape with profiles of leading companies and recent strategic developments
Analysis of emerging technologies and trends shaping future growth
Browse More Trending Research Reports:
Nucleic Acid Extraction Instruments & Reagents Market -
https://www.transparencymarketresearch.com/nucleic-acid-extraction-instruments-reagents-market.html
Oligonucleotide Synthesis Market -
https://www.transparencymarketresearch.com/oligonucleotide-synthesis-market.html
Aptamers Market -
https://www.transparencymarketresearch.com/aptamers-market.html
RNA Therapeutics Market -
https://www.transparencymarketresearch.com/rna-therapeutics-market.html
Small Interfering RNA (siRNA) Therapeutics Market -
https://www.transparencymarketresearch.com/small-interfering-rna-therapeutics-market-report.html
Dark Genome Therapeutics Market -
https://www.transparencymarketresearch.com/dark-genome-therapeutics-market.html
Nucleic Acid Aptamers Market -
https://www.transparencymarketresearch.com/nucleic-acid-aptamers.html
Ligase Enzymes Market -
https://www.transparencymarketresearch.com/ligase-enzymes-market.html
Thermal Cycler Market -
https://www.transparencymarketresearch.com/thermal-cycler-market.html
Electroporation Instruments Market -
https://www.transparencymarketresearch.com/electroporation-instruments-market.html
Legionella Testing Market -
https://www.transparencymarketresearch.com/legionella-testing-market.html
Cell Analysis Market -
https://www.transparencymarketresearch.com/cell-analysis-market.html
Molecular Diagnostics Market -
https://www.transparencymarketresearch.com/molecular-diagnostics-industry.html
Biobanking Market -
https://www.transparencymarketresearch.com/biobanking-market.html
Genetic Testing Services Market -
https://www.transparencymarketresearch.com/genetic-testing-services-market.html
Array Market -
https://www.transparencymarketresearch.com/global-array-market.html
Oligonucleotides Market -
https://www.transparencymarketresearch.com/oligonucleotides-market.html
Digital PCR & Quantitative PCR Market -
https://www.transparencymarketresearch.com/digital-pcr-quantitative-pcr-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website:
https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
AcquisitionLicense out/inOligonucleotide
02 Sep 2025
PITTSBURGH, Sept. 2, 2025 /PRNewswire/ -- RareMed Solutions®, the nation's leader in patient support services for complex therapies, is proud to announce an expansion of its relationship with PTC Therapeutics™ with the launch of non-commercial pharmacy dispensing services for PTC's therapy, Sephience™. The recent approval of Sephience by the FDA marks a significant milestone in the treatment of phenylketonuria (PKU) for both adult and pediatric patients. This new therapy, developed by PTC, has the potential to transform the lives of those living with PKU by offering an innovative treatment option that addresses their unique needs.
Support for Patients and Providers
RareMed will be providing non-commercial pharmacy dispensing services to support patients in challenging circumstances. These services are designed to provide both patients and healthcare providers with appropriate assistance.
To provide seamless access and support, RareMed RareSupport® Team is expanding. A larger team of liaisons will play a crucial role in providing outreach and coordination efforts across PTC's supported therapies so that every patient may receive appropriate, personalized care and attention, while leadership will provide guidance, oversight, and compliance support.
Commitment to Care
RareMed's commitment to providing care extends beyond just the launch of new therapies. The program's support framework is designed to assist patients and providers alike in navigating the treatment landscape with confidence. By expanding the team, RareMed aims to facilitate a smoother, more coordinated experience for all stakeholders involved in the treatment process.
As RareMed continues to grow and adapt to the evolving needs of its patients, the company's focus remains steadfast on delivering compassionate, effective, and timely care.
About Phenylketonuria
Phenylketonuria (PKU) is a rare, inherited metabolic disease, which affects the brain. It is caused by a defect in the gene that helps create the enzyme needed to break down phenylalanine (Phe). If left untreated or poorly managed, Phe—an essential amino acid found in all proteins and most foods—can build up to harmful levels in the body. This causes severe and irreversible disabilities, such as permanent intellectual disability, seizures, delayed development, memory loss, and behavioral and emotional problems. Newborns with PKU initially do not have any symptoms, but symptoms are usually progressive, and damage caused by toxic levels of Phe in the first few years of life is irreversible. Diagnosis of PKU usually takes place during newborn screening programs. There are an estimated 58,000 people living with PKU globally.
About RareMed
RareMed Solutions is the nation's only concierge patient services provider, delivering award-winning care to patients living with complex conditions. RareMed partners with biopharma to transform the lives of patients afflicted with devastating complex conditions by accelerating access to biomedical breakthroughs. RareMed offers case management, co-pay, coupon and financial assistance programs, reimbursement support, nursing support, healthcare professional education, patient adherence & education, and non-commercial pharmacy dispensing services to all fifty states from its headquarters in Pittsburgh, Pennsylvania and bases across the country. RareMed's advanced AI-integrated hub technology offerings, including the revolutionary RarePath® platform, provide unparalleled management and analytics capabilities to biopharma across the nation.
The company has a breadth of experience developing, transitioning, and maintaining therapy-specific solutions that ensure unparalleled manufacturer & patient satisfaction across a variety of patient services models. RareMed's undivided complex disease focus, high caliber associates, fully dedicated teams, and sophisticated proprietary technology enable it to address the unique needs of complex disease patients. RareMed has been consistently recognized as a top workplace in the nation by USA Today and Newsweek and continually raises the benchmark for patient experience according to MMIT patient satisfaction surveys, in which the team recently achieved a perfect 100-point net promoter score. Visit RareMed's website at .
SOURCE RareMed Solutions
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
Drug Approval
02 Sep 2025
Pictured: FDA Headquarters, iStock, Grandbrothers
iStock,
Grandbrothers
Aside from the rare disease market, Novo Nordisk also scored a key regulatory win last month for its blockbuster GLP-1 drug Wegovy, which can now be used to treat patients with metabolic dysfunction-associated steatohepatitis.
August was a big month for the rare disease space, which saw four history-making approvals—though the streak was marred by one rejection. Also walking away with a win last month was Novo Nordisk, which secured a critical label expansion that, according to analysts, could help its blockbuster GLP-1 medication gain access to nearly $2 billion in added peak sales.
Read below for more.
Four Firsts for Rare Diseases
Company: Jazz Pharmaceuticals
Drug: Modeyso
Indication: Glioma
The first rare disease nod came on Aug. 6 for Jazz Pharmaceuticals’ dordaviprone, which was
approved
for patients aged 1 year and above with diffuse midline glioma with an H3 K27M mutation who have progressed after prior treatments. The drug, which will carry the brand name Modeyso, is the first systemic therapy for this specific type of glioma, according to the
FDA’s announcement
of the approval.
Modeyso was approved under the FDA’s accelerated pathway, supported by overall response (OR) data from a pair of Phase I and Phase II studies. According to Jazz’s
news release
announcing the approval, Modeyso achieved an OR of 22% in 50 adult and pediatric patients. Among responders, median duration was 10.3 months. To maintain Modeyso’s approval, Jazz is running a
confirmatory Phase III trial
that is expected to be complete in August 2026.
Company: Insmed
Drug: Brinsupri
Indication: Non-cystic fibrosis bronchiectasis
Not a week after Jazz, Insmed
secured
an FDA nod for brensocatib—now sold as Brinsupri—for the rare respiratory disorser non-cystic fibrosis bronchiectasis. Brinsupri, taken orally once daily, is indicated for patients aged 12 years and up. Insmed has set its price at $88,000 per year—“a little higher” than what analysts were expecting, Guggenheim Partners wrote at the time.
Brinsupri marks two firsts for the biopharma industry: Not only is it the first drug for bronchiectasis to reach the market, but it is also the first approved treatment that works by blocking DPP1, an enzyme that plays a role in activating the inflammatory response in airways.
Brinsupri is backed by data from the Phase III ASPEN trial. Data published in
The
New England Journal of Medicine
in April showed that Brinsupri lowered the rate of pulmonary exacerbations by around 20% versus placebo. The Phase II WILLOW trial also supported Brinsupri’s approval, demonstrating a
roughly 40% reduction
in the risk of exacerbations relative to placebo.
Company:
Precigen
Drug: Papzimeos
Indication: Recurrent respiratory papillomatosis
Then, on Aug. 14, Precigen
won
the FDA’s go-ahead for zopapogene imadenovec-drba, now named Papzimeos, for the treatment of recurrent respiratory papillomatosis (RRP). In its announcement of the approval, the FDA said Papzimeos is a “first-of-its-kind” non-replicating immunotherapy for this disease.
With around 1,000 new cases in the U.S. annually, RRP is a rare disease that manifests as benign tumors in the airways, leading to difficulties in swallowing and breathing. If left unchecked, RRP can lead to death. Papzimeos works by helping the body mount an immune response against cells infected by HPV 6 and HPV 11, both associated with RRP. Pivotal Phase I/II data supported Papzimeos’ approval, showing that the biologic elicited a complete response rate of
more than 50%
in treated patients, while over 85% needed fewer surgical interventions in the year after treatment.
Company:
Ionis Pharmaceuticals
Drug: Dawnzera
Indication: Hereditary angioedema
Capping off the rare disease rally this month is Ionis Pharmaceuticals. On Aug. 22, the California biotech’s antisense oligonucleotide donidalorsen, now branded Dawnzera,
became
the industry’s first RNA-targeting prophylactic for hereditary angioedema (HAE). Patients 12 years and older can receive the therapy, which is given via a subcutaneous injection every four weeks.
Dawnzera targets the prekallikrein mRNA, causing its destruction. This, in turn, reduces overall levels of PKK, a protein that plays a role in swelling and pain attacks in HAE. Data from the OASIS-HAE study backed Thursday’s approval, demonstrating an 81% reduction in HAE attack rate versus placebo over 24 weeks of observation. Results were published May 2024 in the
New England Journal of Medicine
and additionally showed a significant improvement in patients’ quality of life.
Wegovy Win For Novo
On Aug. 15, the FDA
gave the go-ahead
for Novo Nordisk’s blockbuster injection Wegovy to be used in adults with metabolic dysfunction-associated steatohepatitis. The GLP-1 drug, indicated for patients with moderate to advanced liver scarring but without cirrhosis, should be used in conjunction with a reduced-calorie diet and higher physical activity.
Wegovy’s approval in MASH is a “step in the right direction” for Novo, analysts at BMO Capital Markets wrote in an Aug. 17 note to investors, adding that breaking into the MASH market “could start to help shift the momentum” for Wegovy, which in the first half of 2025 has
been hit hard
by the rise of compounders. BMO anticipates peak MASH sales of $1.9 billion for Wegovy.
Data from the Phase III ESSENCE trial, which supported the label expansion,
showed
that Wegovy improved liver fibrosis without worsening steatohepatitis in 37% of treated patients at 72 weeks, versus 22.5% in placebo comparitors. At the same time, Wegovy resolved steatohepatitis without worsening fibrosis in 62.9% of patients, as compared with 34.1% of placebo participants.
Wegovy has “clear efficacy in MASH,” the BMO analysts wrote, adding that its “clean safety pro broad benefits across metabolic disease” could help establish the drug “as a backbone treatment for MASH.”
Wegovy joins Madrigal Pharmaceuticals’ Rezdiffra in the MASH space.
Approved in March 2024
, Rezdiffra
made $180.1 million
last year.
Three COVID-19 Vaccines Cleared, With Restrictions
The FDA on Aug. 27 approved updated COVID-19 vaccines from Pfizer, Moderna and Novavax, but with key limitations: The shots can only be used in adults 65 years and older and younger people who are at elevated risk of severe outcomes.
There are some minor differences across the three, particularly as it pertains to use in the at-risk younger population.
Novavax’s Nuvaxovid, a protein-based shot, is authorized for individuals 12 through 64 years.
Pfizer and BioNTech’s mRNA-based Comirnaty can be given to children as young as 5 years.
Moderna’s mNEXSPIKE is indicated for people 12 through 64 years, while Spikevax is indicated for individuals 6 months through 64 years. Both vaccines are mRNA-based.
These approvals come after Health Secretary Robert F. Kennedy Jr. in May
removed
routine COVID-19 vaccination for healthy children and healthy pregnant women from CDC recommendations.
PTC Hit With Rejection in Friedreich’s Ataxia
The FDA on Aug. 19
turned down
PTC Therapeutics’ vatiquinone, which the company has proposed for the treatment of Friedreich’s ataxia in children and adults.
In its complete response letter, the FDA stated that “substantial evidence of efficacy was not demonstrated” and that PTC would need an additional “adequate and well-controlled study” to support a resubmission. Matthew Klein, the company’s CEO, said in a statement at the time that PTC is planning to meet with the FDA to discuss potential next steps.
Vataquinone is a small molecule that blocks some of the cellular pathways that go awry in patients with frataxin mutations, the underlying cause of Friedreich’s ataxia. Patients with this rare neuromuscular disease suffer from loss of coordination and muscle strength, as well as difficulty speaking, swallowing and breathing. Around 25,000 patients have been diagnosed worldwide, according to PTC.
Vataquinone
missed the mark
in a Phase III, registration-directed trial back in 2023, failing to meet its primary endpoint of improving gait, stability and limb function after 72 weeks. PTC sought registration with the FDA based on secondary outcomes such as stability.
Clinical ResultDrug ApprovalPhase 3Phase 2Vaccine
100 Deals associated with PTC Therapeutics, Inc.
Login to view more data
100 Translational Medicine associated with PTC Therapeutics, Inc.
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 01 Feb 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Discovery
5
7
Preclinical
Phase 1
3
1
Phase 2
Phase 3
1
4
Approved
Other
16
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Ataluren ( DAG1 ) | Muscular Dystrophy, Duchenne More | Approved |
Sepiapterin ( eNOS ) | Phenylketonurias More | Approved |
Eladocagene exuparvovec ( DDC ) | Aromatic Amino Acid Decarboxylase Deficiency More | Approved |
Vatiquinone ( 12/15-LOX ) | Friedreich Ataxia More | Phase 3 |
Votoplam ( HTT x splicing factor ) | Huntington Disease More | Phase 2 |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
