/ Active, not recruitingNot Applicable [Translation] Study on the bioequivalence of sacubitril-valsartan sodium tablets in healthy volunteers
本试验旨在研究健康受试者单次空腹和餐后口服辽宁鑫善源药业有限公司持证、生产的沙库巴曲缬沙坦钠片〔200 mg(沙库巴曲97 mg/缬沙坦103 mg)〕的药代动力学特征;以Novartis Pharma Schweiz AG持证、Novartis Farma S.p.A.生产的沙库巴曲缬沙坦钠片〔诺欣妥®,200 mg(沙库巴曲97 mg/缬沙坦103 mg)〕为参比制剂,比较两制剂中药动学参数Cmax、AUC0-t、AUC0-∞,评价两制剂的人体生物等效性,并观察两制剂在健康受试者中的安全性。
[Translation] This study aimed to study the pharmacokinetic characteristics of a single fasting and postprandial oral administration of sacubitril-valsartan sodium tablets [200 mg (sacubitril 97 mg/valsartan 103 mg)] licensed and produced by Liaoning Xinshanyuan Pharmaceutical Co., Ltd. in healthy subjects; using sacubitril-valsartan sodium tablets [Noxinto®, 200 mg (sacubitril 97 mg/valsartan 103 mg)] licensed by Novartis Pharma Schweiz AG and produced by Novartis Farma S.p.A. as the reference preparation, to compare the pharmacokinetic parameters Cmax, AUC0-t, and AUC0-∞ of the two preparations, to evaluate the human bioequivalence of the two preparations, and to observe the safety of the two preparations in healthy subjects.
/ CompletedNot Applicable [Translation] Study on the bioequivalence of clopidogrel bisulfate tablets in healthy volunteers
本试验旨在研究单次空腹和餐后口服辽宁鑫善源药业有限公司研制、生产的硫酸氢氯吡格雷片(75 mg)的药代动力学特征;以赛诺菲(杭州)制药有限公司持证、生产的硫酸氢氯吡格雷片(波立维®,75 mg)为参比制剂,比较两制剂中药动学参数Cmax、AUC0-t、AUC0-∞,评价两制剂的人体生物等效性。
[Translation] This study aimed to study the pharmacokinetic characteristics of clopidogrel bisulfate tablets (75 mg) developed and produced by Liaoning Xinshanyuan Pharmaceutical Co., Ltd. after single oral administration on an empty stomach or after a meal; using clopidogrel bisulfate tablets (Plavix®, 75 mg) licensed and produced by Sanofi (Hangzhou) Pharmaceutical Co., Ltd. as the reference preparation, the pharmacokinetic parameters Cmax, AUC0-t, and AUC0-∞ of the two preparations were compared, and the bioequivalence of the two preparations in humans was evaluated.
/ CompletedNot Applicable [Translation] Study on the bioequivalence of dapagliflozin tablets in human body
本试验旨在研究单次空腹和餐后口服辽宁鑫善源药业有限公司生产的达格列净片(10 mg)的药代动力学特征;以AstraZeneca Pharmaceuticals LP生产的达格列净片(安达唐®,10 mg)为参比制剂,比较两制剂中药动学参数Cmax、AUC0-t、AUC0-∞,评价两制剂的人体生物等效性。
[Translation] This study aimed to investigate the pharmacokinetic characteristics of dapagliflozin tablets (10 mg) produced by Liaoning Xinshanyuan Pharmaceutical Co., Ltd. after single oral administration on an empty stomach or after a meal; using dapagliflozin tablets (Andatang®, 10 mg) produced by AstraZeneca Pharmaceuticals LP as the reference preparation, the pharmacokinetic parameters Cmax, AUC0-t, and AUC0-∞ of the two preparations were compared, and the bioequivalence of the two preparations in humans was evaluated.
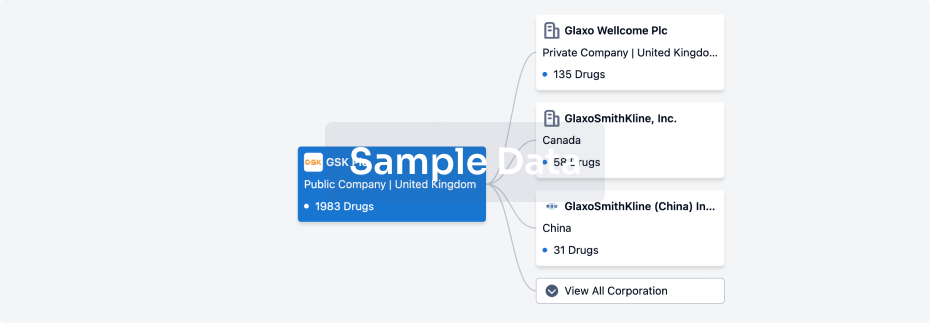
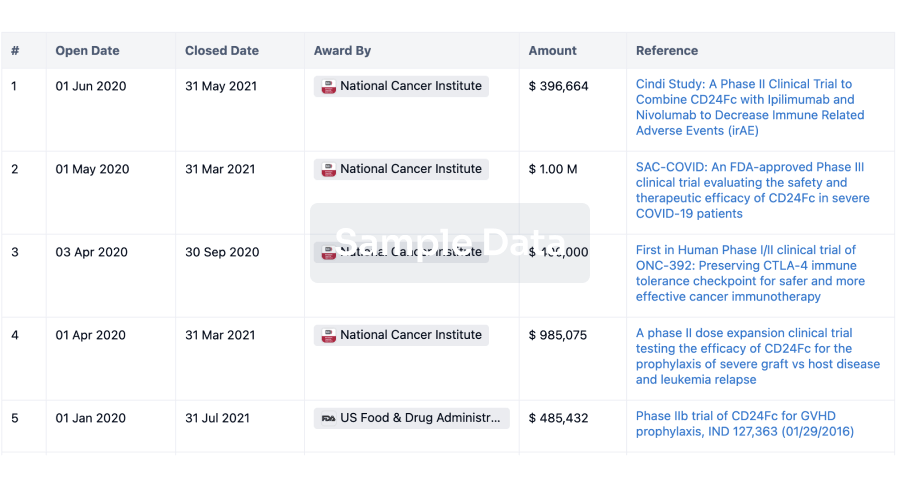
100 Clinical Results associated with Chaoyang Deyuan Pharmaceutical Co., Ltd.
0 Patents (Medical) associated with Chaoyang Deyuan Pharmaceutical Co., Ltd.
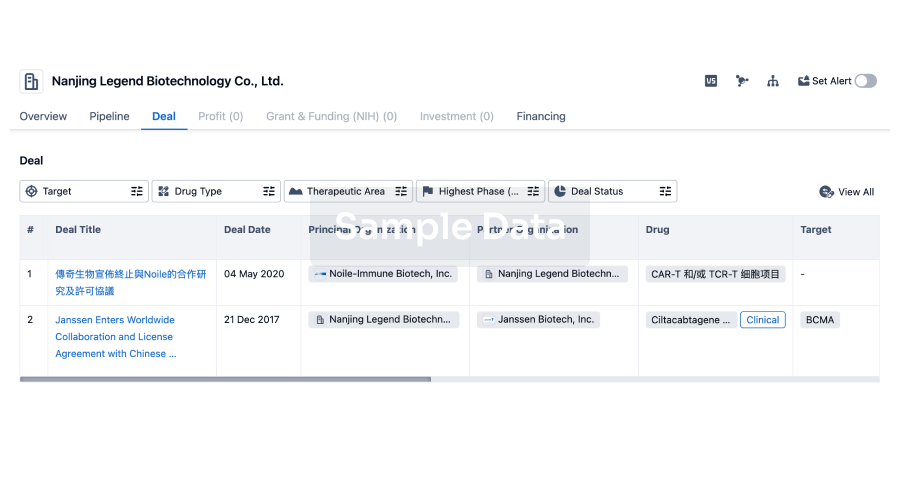
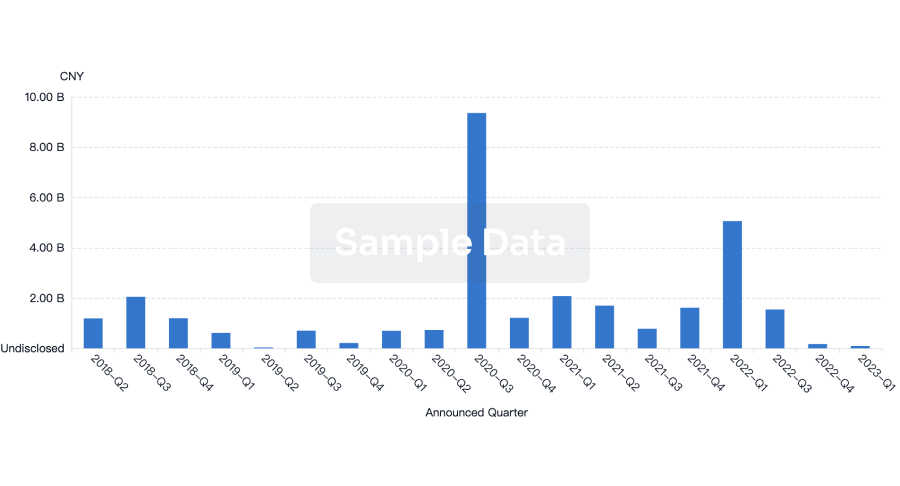
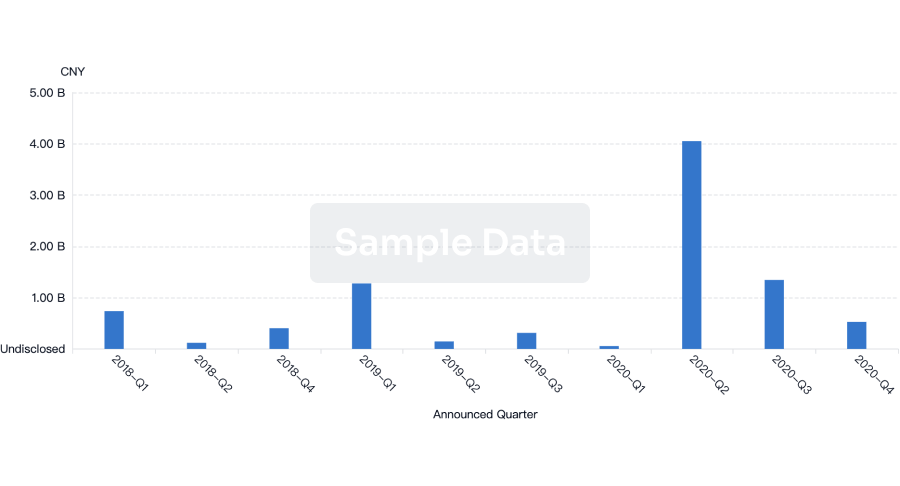
100 Deals associated with Chaoyang Deyuan Pharmaceutical Co., Ltd.
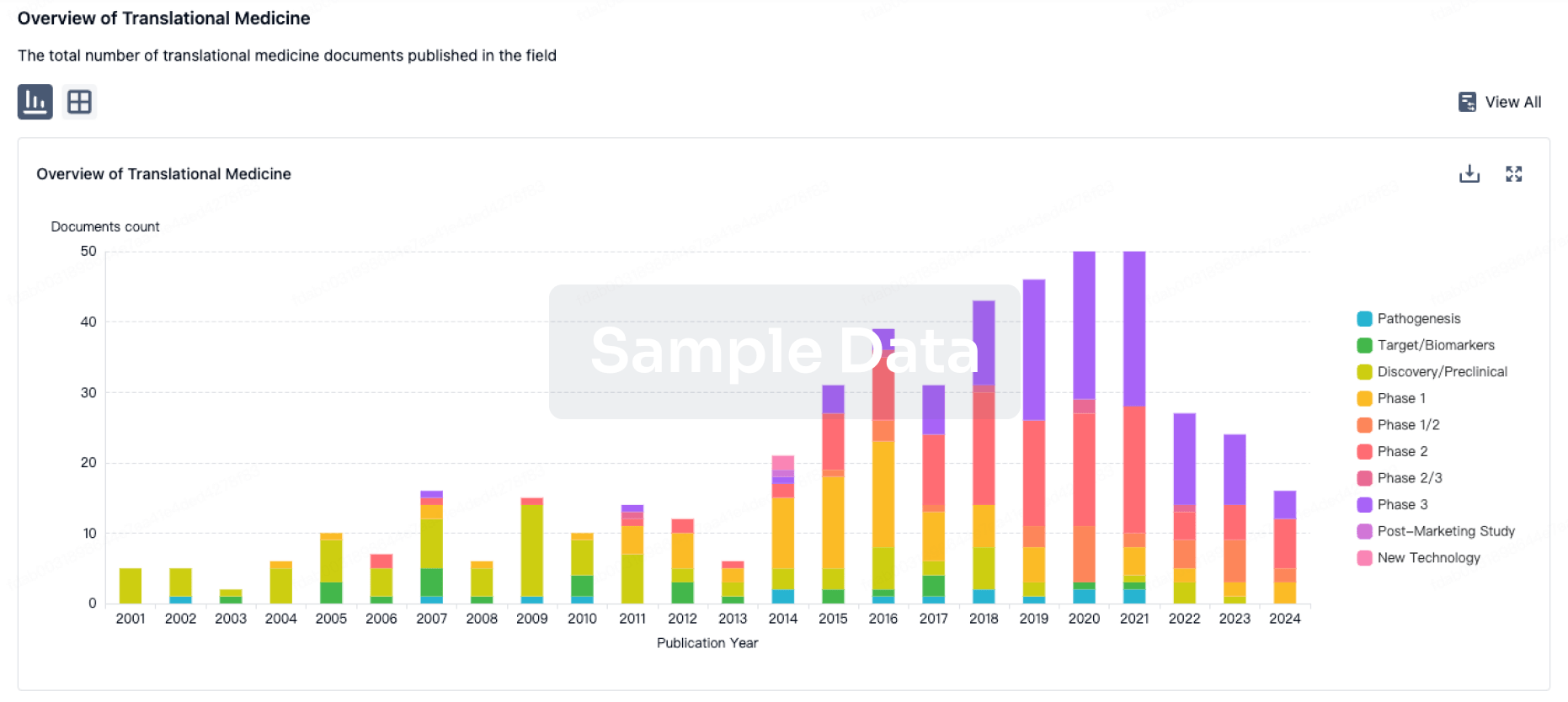
100 Translational Medicine associated with Chaoyang Deyuan Pharmaceutical Co., Ltd.