Optimize Your Synapse Experience: A Step-by-Step Guide to Searching Fluticasone Propionate
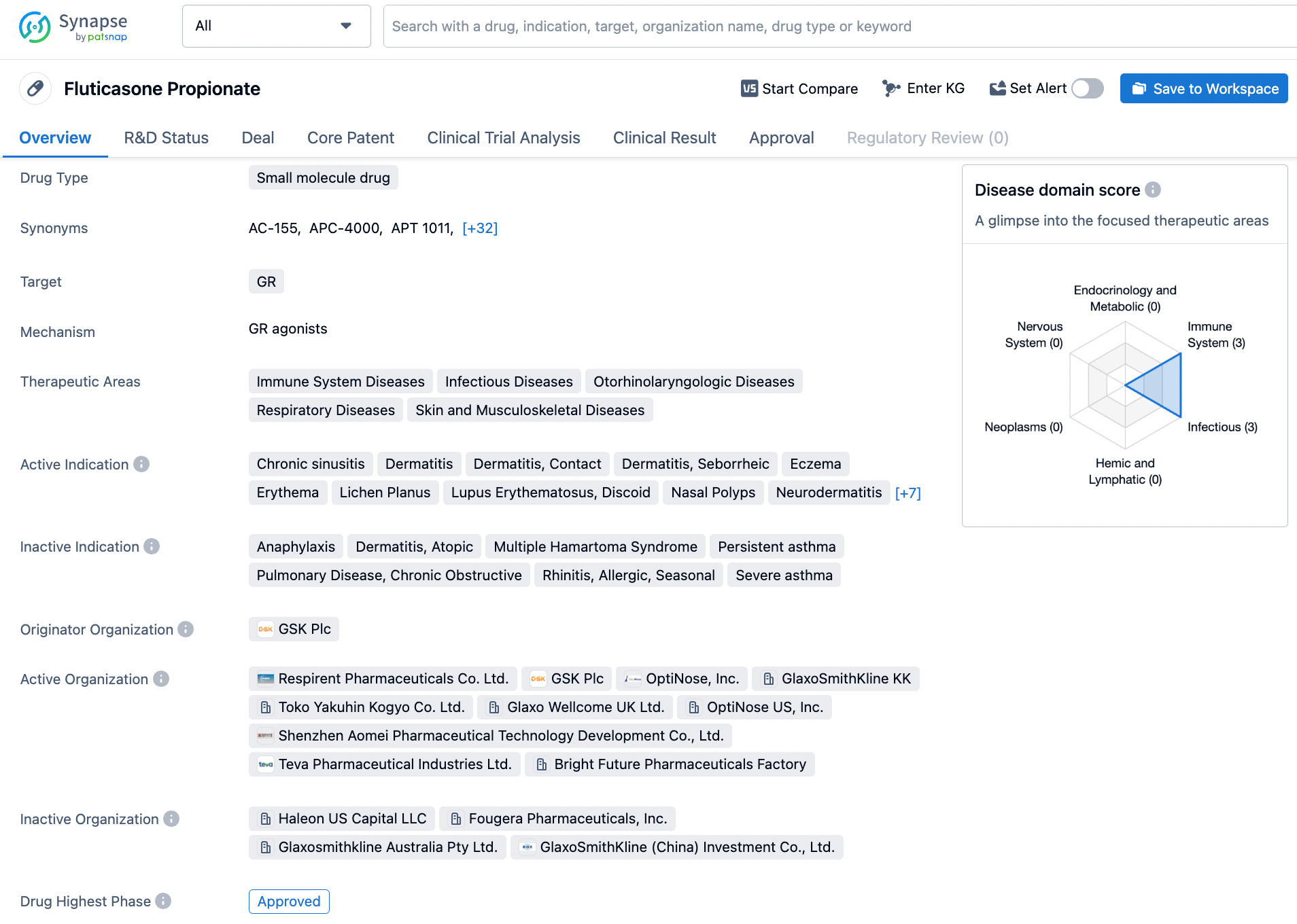
Fluticasone Propionate, a small molecule drug, targets the glucocorticoid receptor and acts as an agonist. The drug has been approved for the treatment of various dermatological conditions such as dermatitis, contact dermatitis, seborrheic dermatitis, eczema, erythema, lichen planus, and discoid lupus erythematosus, among others. Additionally, it has been approved for the treatment of nasal polyps, neurodermatitis, and prurigo. However, the drug's mechanism of action remains unknown. GSK is the originator organization of fluticasone propionate, and it was first approved for use in December 1990. Fluticasone propionate has potent anti-inflammatory and immunosuppressive effects due to the activation of the glucocorticoid receptor, which make it effective in treating the symptoms associated with these conditions. Nevertheless, like all medications, fluticasone propionate has potential side effects such as adrenal suppression, increased risk of infections, and osteoporosis, among others, and should be used under the guidance of a healthcare provider. Click on the image below to begin the exploration journey of Fluticasone Propionate through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!