CheckMate -9DW Success: Opdivo and Yervoy Boost Survival in Progressive Liver Cancer
Bristol Myers Squibb has disclosed that the Phase 3 study named CheckMate -9DW, which assesses the combination of Opdivo (nivolumab) with Yervoy (ipilimumab) as an initial therapy in individuals suffering from progressive hepatocellular carcinoma with no previous systemic therapy history, achieved its principal objective of enhancing overall survival relative to the selected treatments of sorafenib or lenvatinib, as determined during an early planned review.
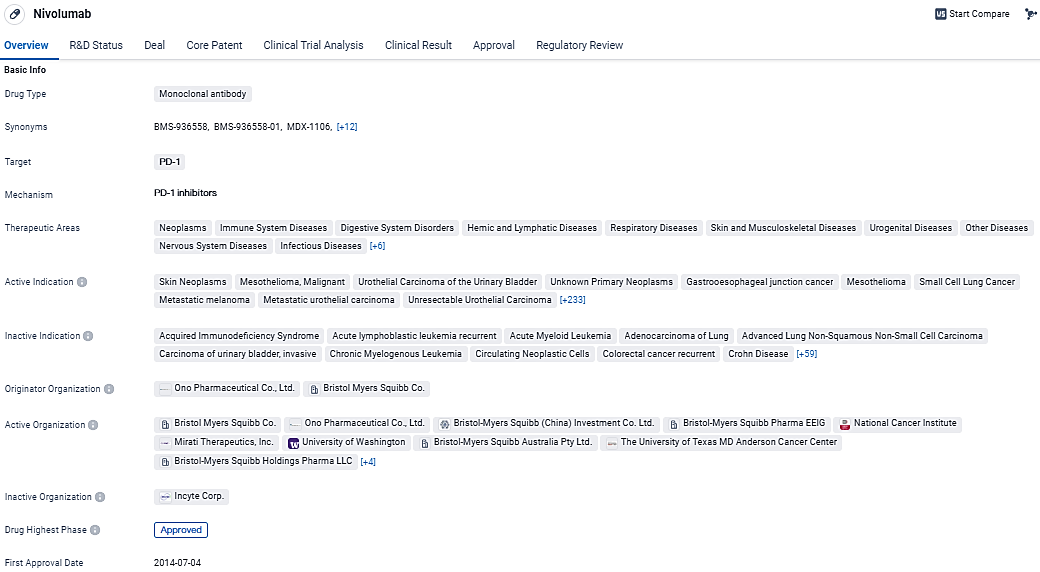
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The combination therapy involving Opdivo and Yervoy has shown a notable and substantial extension in overall survival (OS) rates when measured up against the standard choice therapies of sorafenib or lenvatinib, with statistically valuable results. The combined treatment's safety profile coincides with findings from earlier studies, showing a predictable reaction that aligns with pre-existing treatment guidelines without uncovering any unforeseen safety concerns.
"Patients with liver cancer in its latter stages continue to require more therapeutic interventions that might positively impact their longevity," remarked Dana Walker, M.D., M.S.C.E., who is the vice president overseeing the development of treatments for gastrointestinal and genitourinary cancers at Bristol Myers Squibb.
Walker added, "The survival advantage seen with the Opdivo-Yervoy regimen in the CheckMate -9DW research underscores its capacity to enhance patient outcomes when compared to established TKI therapies."
The organization plans to conduct a thorough review of the study’s findings and intends to present this information to the medical community at an upcoming healthcare meeting. Additionally, discussions will occur with regulatory bodies concerning the findings.
Opdivo functions as an inhibitor targeting the PD-1 immune checkpoint, a pioneering approach that empowers the immune system's inherent abilities to counteract cancerous cells. By leveraging the body's immune defenses against cancer, Opdivo has taken a critical role in the management of various cancer types.
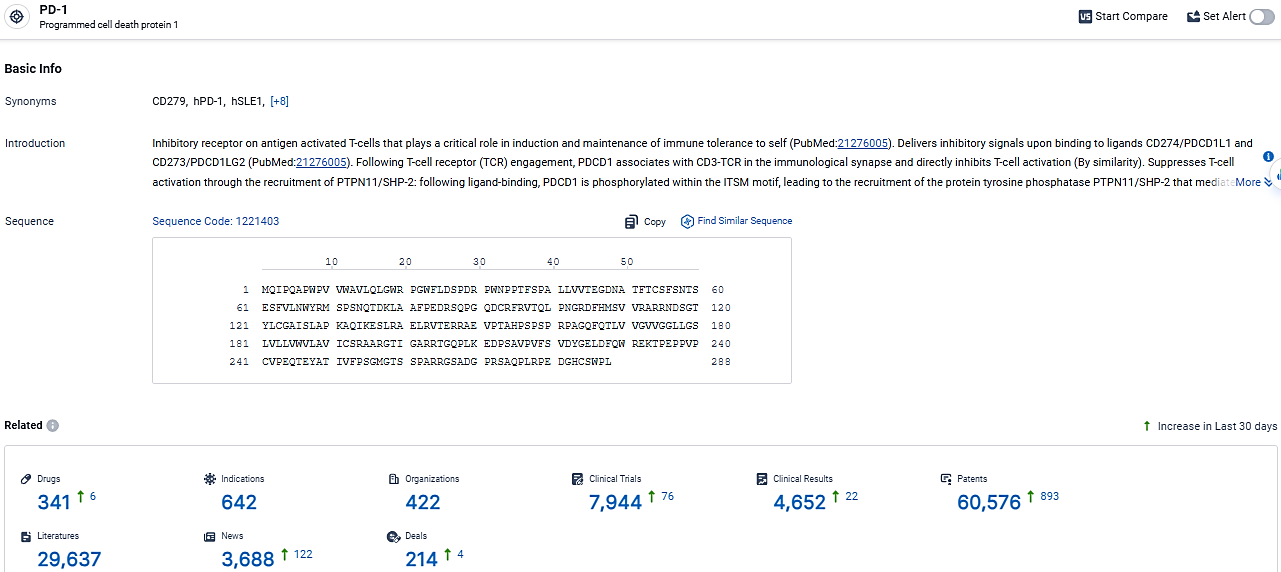
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 21, 2024, there are 341 investigational drugs for the PD-1 target, including 642 indications, 422 R&D institutions involved, with related clinical trials reaching 7944, and as many as 60574 patents.
Opdivo has shown promising results in treating various diseases, and its approval in multiple countries highlights its potential as a valuable therapeutic option. However, careful market analysis and strategic planning will be necessary to navigate the competitive landscape and maximize the drug's commercial success.