4DMT Highlights Promising Clinical Results for 4D-150 and Reveals Phase 3 Strategy at Wet AMD Program Event
4D Molecular Therapeutics (Nasdaq: FDMT, also known as 4DMT or the Company), a prominent clinical-stage genetic medicines enterprise dedicated to maximizing the capabilities of genetic treatments for widespread diseases, has reported data demonstrating sustained robust and prolonged clinical efficacy. These findings are derived from the latest interim follow-up results of the Phase 1/2 PRISM clinical trial, as well as the design of the Phase 3 4FRONT study, all of which will be showcased during the 4D-150 Wet AMD Development Day.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are steadfast in advancing support for 4D-150, fortified by promising interim results from PRISM, highlighting a significant reduction in overall treatment burden and potential long-term clinical benefits in previously treated patients. The emerging safety profile is on par with existing anti-VEGF agents,” stated David Kirn, M.D., Co-founder and CEO of 4DMT.
“Our exceptional senior leadership team, with extensive late-stage drug development, regulatory, and commercial expertise in high-demand ophthalmology markets, is prepared to design and implement our forthcoming pivotal studies. We anticipate launching 4FRONT-1, our inaugural Phase 3 study of 4D-150 for wet AMD, in the first quarter of 2025, and we are eager to continue progressing this potentially transformative candidate that addresses the limitations of current treatments for wet AMD.”
"As responsible developers, we initiated clinical development for 4D-150 with severe wet AMD patients in the Phase 1/2a segment of PRISM. After noting positive tolerability and clinical outcomes, we, along with our investigators, are confident in 4D-150’s potential for a broader spectrum of wet AMD patients, leading to the design of the Phase 2b cohort," said Robert Kim, M.D., Chief Medical Officer at 4DMT. "Interim data from Phase 2b indicate strong clinical activity, especially in patients more recently diagnosed."
4D-150 Wet AMD Development Day Key Updates:
Encouraging Interim Data from the 4D-150 Phase 1/2 PRISM Study:
Clinical Activity (data cutoff: September 3, 2024):
Significant and sustained reduction in treatment burden was noted in all PRISM cohorts using the planned Phase 3 dosage of 3E10 vg/eye of 4D-150 (*using Kaplan-Meier endpoint calculations with 52-week follow-up for Phase 1/2a and varied 32–52 week follow-up for Phase 2b; **diagnosed ≤6 months).
Phase 1/2a Severe Group (n=24, at 52 weeks):
83% reduction in annualized injections
52% had 0 or 1 injection*
44% were injection-free*
Phase 2b Broad Group (n=30, at 52 weeks):
89% reduction in annualized injections
80% had 0 or 1 injection*
70% were injection-free*
Phase 2b Recently Diagnosed** Group (n=15, at 52 weeks):
98% reduction in annualized injections
100% had 0 or 1 injection*
87% were injection-free*
Central Subfield Thickness (CST): maintained anatomical control with reduced fluctuations
Mean best-corrected visual acuity (BCVA): stable (Phase 1/2a) or improved (Phase 2b)
Safety (data cutoff: August 23, 2024):
4D-150 continues to exhibit favorable tolerability
The rate of 4D-150–related intraocular inflammation (IOI) is comparable to those reported for approved anti-VEGF agents
Wet AMD:
2.8% (2 out of 71) experienced 4D-150-related IOI at any point
2 patients had transient 1+ vitreous cells
99% (70 out of 71) completed steroid prophylaxis taper on schedule
97% (69 out of 71) remained steroid-free
Diabetic Macular Edema (DME; SPECTRA trial):
No patients treated with any dose (n=22) have experienced IOI events at any time
No occurrences of 4D-150-related hypotony, endophthalmitis, vasculitis, choroidal effusions, or retinal artery occlusions have been observed in both the wet AMD and DME programs.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
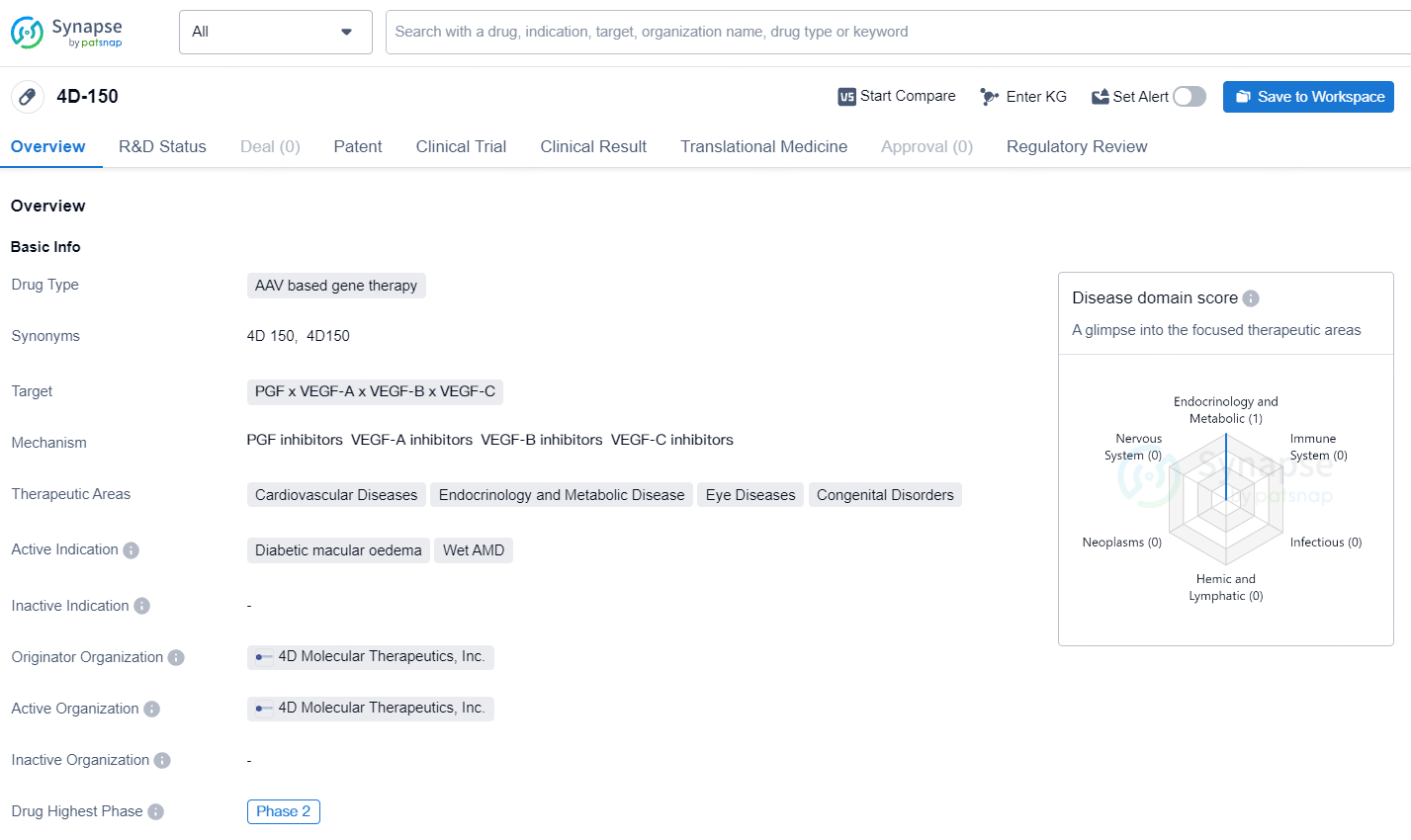
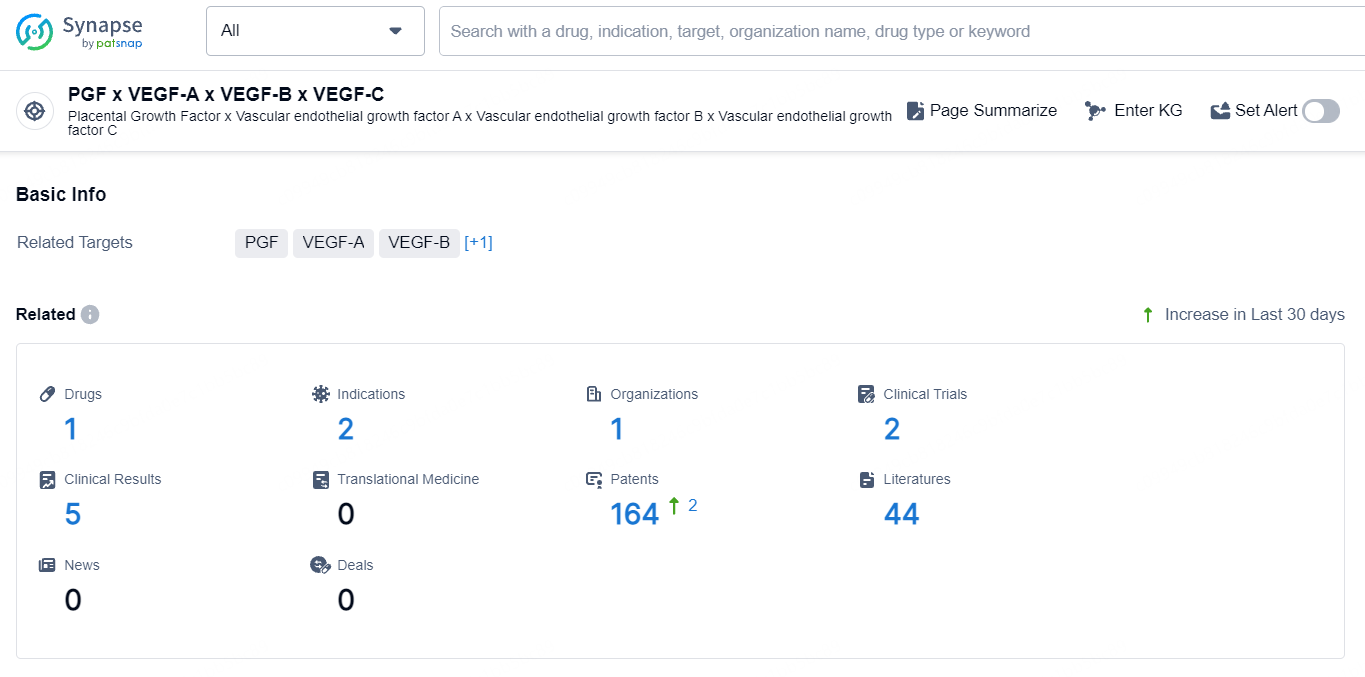
According to the data provided by the Synapse Database, As of September 23, 2024, there are 1 investigational drug for the PGF, VEGF-A, VEGF-B, and VEGF-C target, including 2 indications, 1 R&D institution involved, with related clinical trial reaching 2, and as many as 164 patents.
4D-150 is an AAV based gene therapy drug developed by 4D Molecular Therapeutics, Inc. This drug targets PGF, VEGF-A, VEGF-B, and VEGF-C and is intended for the treatment of cardiovascular diseases, endocrinology and metabolic diseases, eye diseases, and congenital disorders. The active indications for 4D-150 include diabetic macular oedema and wet AMD. 4D-150 has reached Phase 2 of clinical trials, which is the highest phase globally. The drug is classified as a Regenerative Medicine Advanced Therapy and has also received PRIME designation.