A Comprehensive Review of Spiramycin's R&D Innovations and Drug Target Mechanism
Spiramycin's R&D Progress
Spiramycin is a small molecule drug that primarily targets the 50S subunit. It has been approved for use in various therapeutic areas, including infectious diseases, eye diseases, hemic and lymphatic diseases, mouth and tooth diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, otorhinolaryngologic diseases, and other diseases.
Spiramycin is indicated for the treatment of toxoplasmosis, protozoan infections, acute bronchitis, adnexitis, bacterial infections, etc.
Spiramycin received its first approval in 1955 in France, making it a well-established drug in the market. It is classified as an orphan drug, indicating that it is used to treat rare diseases or conditions. This classification often comes with certain regulatory benefits, such as extended market exclusivity and financial incentives.
The highest phase of development for Spiramycin is approved globally. This means that the drug has successfully completed clinical trials and has been deemed safe and effective for its indicated uses. The approval status in both regions suggests that Spiramycin has met the necessary regulatory requirements and can be marketed and sold to patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Spiramycin: 50S subunit inhibitors
From a biomedical perspective, 50S subunit inhibitors are a type of drugs that target the 50S subunit of bacterial ribosomes. Ribosomes are essential cellular structures responsible for protein synthesis. In bacteria, ribosomes consist of two subunits, the 30S and 50S subunits. The 50S subunit is involved in the catalytic activity of the ribosome.
50S subunit inhibitors work by binding to the 50S subunit and interfering with the normal functioning of the ribosome. By doing so, they inhibit bacterial protein synthesis, ultimately leading to the inhibition of bacterial growth and reproduction. These inhibitors are commonly used as antibiotics to treat bacterial infections.
Examples of 50S subunit inhibitors include macrolides (such as erythromycin and clarithromycin), lincosamides (such as clindamycin), and streptogramins (such as quinupristin/dalfopristin). These drugs are effective against a wide range of bacterial pathogens and are commonly used in the treatment of respiratory tract infections, skin and soft tissue infections, and certain sexually transmitted infections.
It's important to note that 50S subunit inhibitors specifically target bacterial ribosomes and do not affect human ribosomes, making them selective for bacterial infections.
Drug Target R&D Trends for Spiramycin
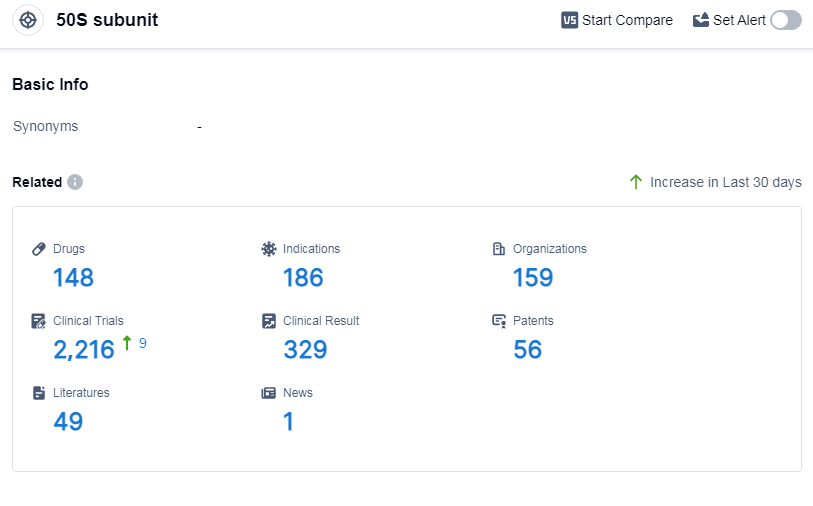
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 148 50S subunit drugs worldwide, from 159 organizations, covering 186 indications, and conducting 2216 clinical trials.
The analysis of the target 50S subunit reveals a competitive landscape with multiple companies focusing on its development. Pfizer Inc. leads in terms of the highest stage of development, with several approved drugs. Bacterial Infections is the most common indication for drugs targeting the 50S subunit. Small molecule drugs are progressing rapidly, indicating intense competition around innovative drugs. China is the fastest-developing country in this target, with a significant number of approved drugs. The future development of the target 50S subunit holds promise for the pharmaceutical industry, with potential advancements in the treatment of various indications and the introduction of innovative drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Spiramycin is a widely used small molecule drug that targets the 50S subunit. It has a broad range of therapeutic applications, particularly in the treatment of various infectious diseases. With its long history of approval and orphan drug status, Spiramycin holds a significant position in the pharmaceutical industry.