Decoding ruxolitinib phosphate: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Ruxolitinib phosphate's R&D Progress
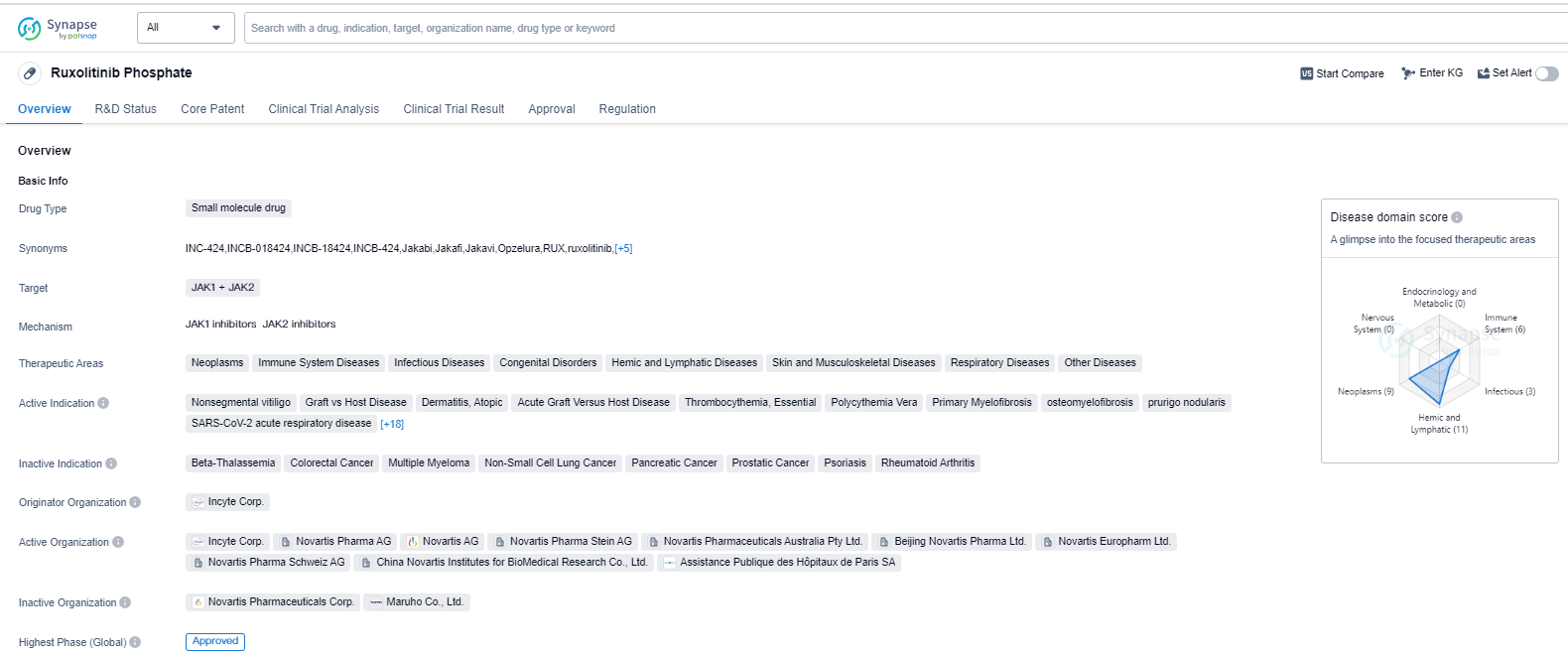
Ruxolitinib Phosphate is a small molecule drug that targets JAK1 and JAK2, making it a potential treatment option for various therapeutic areas. These areas include neoplasms, immune system diseases, infectious diseases, congenital disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, respiratory diseases, and other diseases.
The drug has shown efficacy in treating several indications, including nonsegmental vitiligo, graft vs host disease, dermatitis (atopic and seborrheic), acute graft versus host disease, thrombocythemia (essential), polycythemia vera, primary myelofibrosis, osteomyelofibrosis, prurigo nodularis, SARS-CoV-2 acute respiratory disease (COVID-19), cytokine release syndrome, chronic eczema, lymphohistiocytosis (hemophagocytic), anemia, chronic myelogenous leukemia, bronchiolitis obliterans, head and neck neoplasms, breast cancer, leukemia (acute lymphoblastic and chronic lymphocytic), alopecia areata, B-cell chronic lymphocytic leukemia, thalassemia, cachexia, and Hodgkin's lymphoma.
The drug was developed by Incyte Corp., and it has received approvals in both the United States and China. The first approval for Ruxolitinib Phosphate was granted in November 2011 in the United States. It is worth noting that the drug has undergone accelerated assessment and has been designated as an orphan drug, indicating its potential to address unmet medical needs.
Ruxolitinib Phosphate's approval in multiple therapeutic areas highlights its versatility and potential to address various diseases. Its mechanism of action targeting JAK1 and JAK2 suggests its potential in modulating immune responses and inhibiting abnormal cell growth.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for ruxolitinib phosphate: JAK1 inhibitors JAK2 inhibitors
JAK1 inhibitors and JAK2 inhibitors are types of drugs that target specific enzymes called Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2), respectively. These enzymes are involved in the signaling pathways of cytokines, which are small proteins that play a crucial role in cell communication and immune response.
From a biomedical perspective, JAK1 and JAK2 inhibitors are used in the treatment of various inflammatory and autoimmune diseases, such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. By inhibiting the activity of these enzymes, these drugs can help regulate the immune response and reduce inflammation.
JAK1 and JAK2 inhibitors work by binding to the enzymes and blocking their activity. This prevents the downstream signaling of cytokines and reduces the production of pro-inflammatory molecules. By modulating the immune response, these inhibitors can alleviate the symptoms of inflammatory diseases and improve patient outcomes.
It's important to note that JAK1 inhibitors specifically target JAK1 enzyme, while JAK2 inhibitors specifically target JAK2 enzyme. Although they belong to the same class of drugs, their specificity allows for more targeted therapeutic effects depending on the disease being treated.
Drug Target R&D Trends for ruxolitinib phosphate
JAK1 and JAK2 are enzymes belonging to the Janus kinase family, playing crucial roles in the human body. They are involved in the signaling pathways of various cytokines and growth factors, regulating important cellular processes. JAK1 and JAK2 are primarily responsible for transmitting signals from cell surface receptors to the nucleus, thereby influencing gene expression and cellular functions. Dysregulation of JAK1 and JAK2 activity has been implicated in several diseases, including autoimmune disorders and certain types of cancers. Targeting these enzymes with specific inhibitors has emerged as a promising therapeutic approach in the pharmaceutical industry, aiming to modulate their activity and restore normal cellular function.
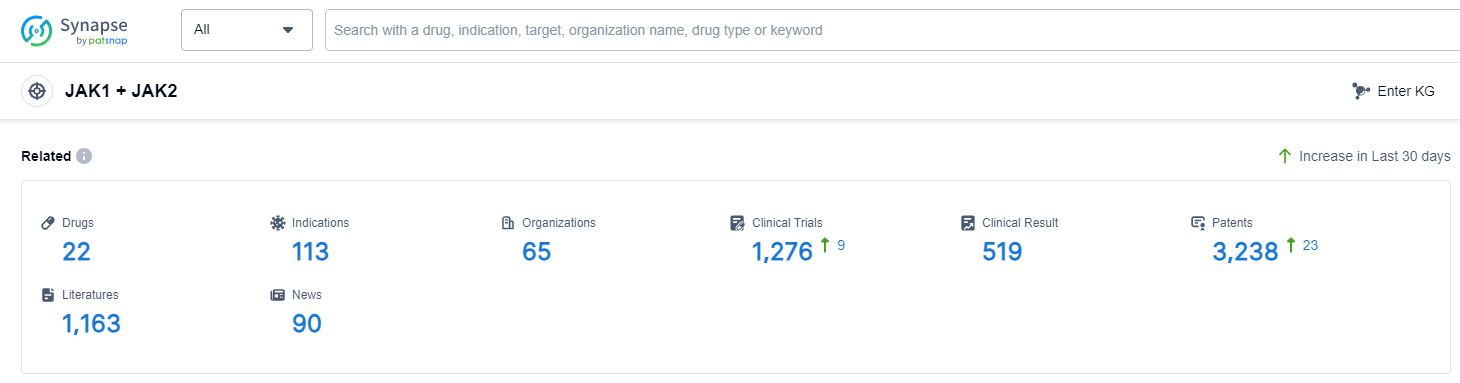
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 22 JAK1 + JAK2 drugs worldwide, from 65 organizations, covering 113 indications, and conducting 1276 clinical trials.
The analysis of the current competitive landscape of target JAK1 + JAK2 reveals that Novartis AG is the leading company with the highest stage of development. The indication analysis shows that several drugs have been approved for relevant indications, including Rheumatoid Arthritis, Dermatitis, Atopic, Arthritis, Psoriatic, Colitis, Ulcerative, and others. The drug types progressing most rapidly are Small molecule drugs and Chemical drugs. The country/location analysis indicates that Japan, China, United States, and European Union are developing fastest under this target. China has shown progress in the development of drugs, with several companies involved in different phases of development. Overall, the target JAK1 + JAK2 has a competitive landscape with multiple companies and countries actively involved in research and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Ruxolitinib Phosphate's approval in multiple indications and its designation as an orphan drug demonstrate its potential as a valuable treatment option in the pharmaceutical industry. Its broad therapeutic areas and successful regulatory assessments position it as a significant player in the field of biomedicine.