A Rising Force in Tumor Immunotherapy: PD-1 Inhibitors
Programmed cell death protein-1 (PD-1) is an important immune inhibitory molecule, belonging to the immunoglobulin superfamily. It is a membrane protein with 288 amino acid residues, initially cloned from the apoptotic mouse T-cell hybridoma 2B4.11. The immunoregulation targeting PD-1 has great significance in tumor antagonism, anti-infection, anti-autoimmune diseases, and organ transplant survival.
The primary function of PD-1 is to limit the activity of peripheral tissue T cells in inflammatory responses caused by infection, and inhibit autoimmune reactions. PD-1 can be expressed on T cells, B cells, natural killer T cells, activated monocytes, and dendritic cells (DC). The ligands of PD-1 have been identified as PD-L1 and PD-L2, with PD-L1 (Programmed Death Ligand 1), being its main ligand, its expression is upregulated in various solid tumors, leading to a decrease in function of local tumor-infiltrating CD4+CD8+T cells and cytokine production.
PD-1 is a key marker of T cell exhaustion. Under normal physiological conditions, PD-1 signaling is strictly regulated. Its pathway is an immune regulatory mechanism that can regulate the intensity and breadth of immune responses, to prevent normal tissue from damage and destruction. In the tumor microenvironment (TME), the binding of PD-1 expressed on effector T cells and PD-L1 expressed on tumor cells leads to dephosphorylation of TCR proximal signaling molecules, which attenuates TCR and CD28 signaling and ultimately promotes T cell apoptosis, anergy, and functional exhaustion, allowing tumor cells to escape the immune system's killing.
Many tumor cells express PD-L1, and inhibiting the interaction between PD-1 and PD-L1 can enhance T-cell responses in vitro and mediate clinical anti-tumor activity. This process is called immune checkpoint blockade. The high expression of PD-L1 in tumor cells and its binding with PD-1 on the surface of immune T cells inhibits the body's immune response, promoting tumor progression and metastasis. The combined treatment of PD-1 and anti-CTLA4 has become an important tumor treatment in the field of checkpoint inhibitors. Currently, inhibitors are used clinically to block the PD-1/PD-L1 signaling pathway, restoring the body's immune response to tumor cells, and achieving the effect of tumor immunotherapy.
PD-1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 311 PD-1 drugs worldwide, from 341 organizations, covering 286 indications, and conducting 7782 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target PD-1 reveals a competitive landscape with several companies actively developing PD-1 inhibitors. Akeso, Inc., Bristol Myers Squibb Co., and Merck & Co., Inc. are the leading companies in terms of R&D progress.
The most common indications for PD-1 inhibitors include Hodgkin's Lymphoma, Non-Small Cell Lung Cancer, Melanoma, Esophageal Squamous Cell Carcinoma, and Hepatocellular Carcinoma. Monoclonal antibodies are the dominant drug type, followed by bispecific antibodies and biosimilars.
China, the United States, and the European Union are the fastest-developing countries/locations in the PD-1 inhibitor market. The future development of PD-1 inhibitors will depend on the success of ongoing clinical trials, competition among different drug types, and regulatory dynamics in different countries.
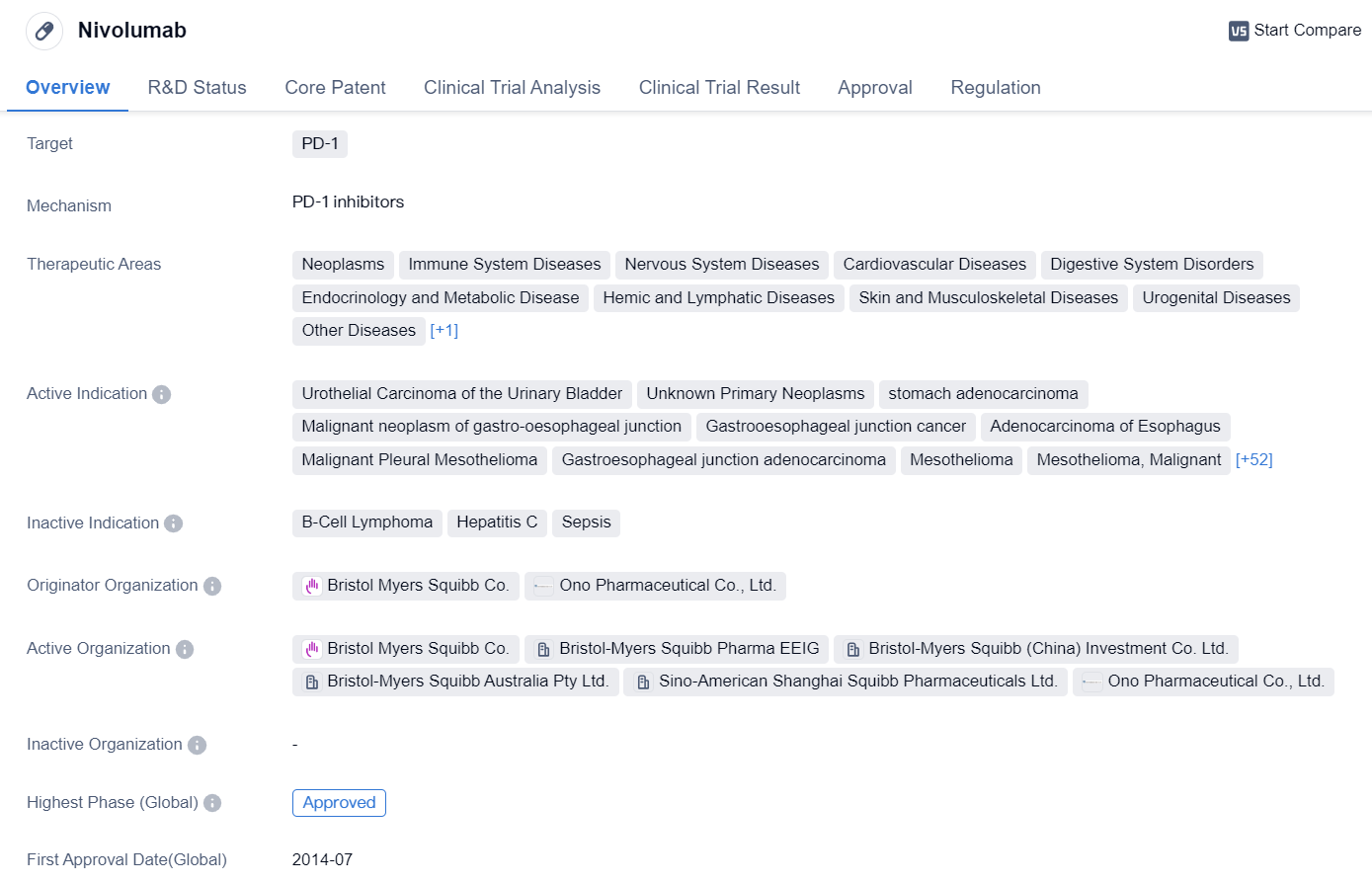
Key Drug: Nivolumab
Nivolumab is a monoclonal antibody drug that targets PD-1, a protein found on immune cells. It has been approved for use in various therapeutic areas, including neoplasms (tumors), immune system diseases, nervous system diseases, cardiovascular diseases, digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, and respiratory diseases.
The drug has shown efficacy in treating several types of cancers, such as urothelial carcinoma of the urinary bladder, stomach adenocarcinoma, malignant neoplasm of the gastro-oesophageal junction, and hepatocellular carcinoma. It has also been approved for use in treating other cancers like colorectal cancer, head and neck neoplasms, renal cell carcinoma, non-small cell lung cancer, melanoma, breast cancer, bladder cancer, liver cancer, small cell lung cancer, pancreatic cancer, and uterine cervical cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Nivolumab was first approved in Japan in July 2014 and has since received approvals in other countries. It is developed by Bristol Myers Squibb Co. and Ono Pharmaceutical Co., Ltd. The drug has reached the highest phase of approval globally and in China.
In terms of regulations, nivolumab has been granted priority review, accelerated approval, fast track designation, orphan drug status, and breakthrough therapy designation. These regulatory designations highlight the potential of the drug to address unmet medical needs and expedite its development and approval process.
Overall, nivolumab is a monoclonal antibody drug that targets PD-1 and has shown promising results in treating various types of cancers. Its wide range of therapeutic areas and multiple regulatory designations indicate its potential as a significant treatment option in the field of biomedicine.
Pembrolizumab
Pembrolizumab is a monoclonal antibody drug that targets PD-1, a protein found on immune cells. It has been approved for use in various therapeutic areas, including neoplasms (tumors), urogenital diseases, respiratory diseases, digestive system disorders, endocrinology and metabolic diseases, immune system diseases, hemic and lymphatic diseases, infectious diseases, nervous system diseases, eye diseases, mouth and tooth diseases, skin and musculoskeletal diseases, otorhinolaryngologic diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several types of cancer, including HER2-negative breast cancer, microsatellite instability cancer, uterine neoplasms, breast cancer, HER2 positive gastroesophageal junction adenocarcinoma, HER2-positive gastric cancer, colorectal cancer, triple negative breast cancer, esophageal squamous cell carcinoma, squamous cell carcinoma, cancer with a high tumor mutational burden, cutaneous squamous cell carcinoma, microsatellite instability-high colorectal cancer, squamous non-small cell lung cancer, endometrial carcinoma, esophageal carcinoma, small cell lung cancer, renal cell carcinoma, metastatic non-squamous non-small cell lung carcinoma, squamous cell carcinoma of the head and neck, hepatocellular carcinoma, Merkel cell carcinoma, mediastinal large B-cell lymphoma, uterine cervical cancer, stomach cancer, microsatellite instability-high solid tumors, mismatch repair-deficient solid tumors, microsatellite instability-high cancer, transitional cell carcinoma, Hodgkin's lymphoma, classical Hodgkin's lymphoma, recurrent squamous cell carcinoma of the head and neck, head and neck neoplasms, non-small cell lung cancer, melanoma, locally advanced cholangiocarcinoma, metastatic biliary tract carcinoma, unresectable biliary tract carcinoma, biliary tract neoplasms, small intestine carcinoma, metastatic hormone-sensitive prostate cancer, bile duct neoplasms, endometrioid carcinoma, liver cancer, prostatic cancer, ER-positive/HER2-negative breast cancer, bladder cancer, solid tumors, gastroesophageal junction adenocarcinoma, adenocarcinoma, nasopharyngeal neoplasms, adenocarcinoma of the esophagus, carcinoma, malignant pleural mesothelioma, sinonasal undifferentiated carcinoma, differentiated thyroid gland carcinoma, high-grade serous adenocarcinoma of the ovary, primary peritoneal carcinoma, marginal zone B-cell lymphoma, hormone receptor-positive HER2-negative breast cancer, acute myeloid leukemia, peritoneal neoplasms, leukoplakia, fallopian tube carcinoma, B-cell lymphoma, non-Hodgkin lymphoma, malignant ascites, gastrointestinal neoplasms, uveal melanoma, meningioma, myelodysplastic syndromes, ovarian cancer, germ cell and embryonal neoplasms, osteosarcoma, meningeal carcinomatosis, adenoid cystic carcinoma, penile neoplasms, rectal cancer, recurrent respiratory papillomatosis, anus neoplasms, carcinoid tumor, cholangiocarcinoma, mesothelioma, parotid neoplasms, thyroid cancer, vulvar neoplasms, Richter's syndrome, diffuse large B-cell lymphoma, follicular lymphoma, inflammatory breast neoplasms, lymphoma, hematologic neoplasms, leiomyosarcoma, T-cell lymphoma, mantle-cell lymphoma, acute lymphoblastic leukemia, pancreatic cancer, and muscle invasive bladder carcinoma.
Pembrolizumab was first approved in the United States in September 2014 by Merck & Co., Inc. It has also received approvals in other countries, including China. The drug has undergone priority review, accelerated approval, and has been designated as an orphan drug and breakthrough therapy.
In summary, pembrolizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various cancers and other diseases. Its approval in multiple therapeutic areas and its designation as a breakthrough therapy highlight its potential in improving patient outcomes.