Analysis on the Research Progress of PDE4 Inhibitors
Phosphodiesterase 4 (PDE4) is an enzyme found in various tissues of the human body, including the immune cells, brain, and lungs. It plays a crucial role in regulating the levels of cyclic adenosine monophosphate (cAMP), a signaling molecule involved in numerous cellular processes. PDE4 specifically breaks down cAMP, thereby controlling its concentration and influencing various physiological functions. In the immune system, PDE4 inhibition can reduce the production of inflammatory mediators, making it a target for anti-inflammatory drugs. Additionally, PDE4 inhibitors have shown potential in treating respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD) by relaxing airway smooth muscles.
PDE4 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 174 PDE4 drugs worldwide, from 175 organizations, covering 122 indications, and conducting 717 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.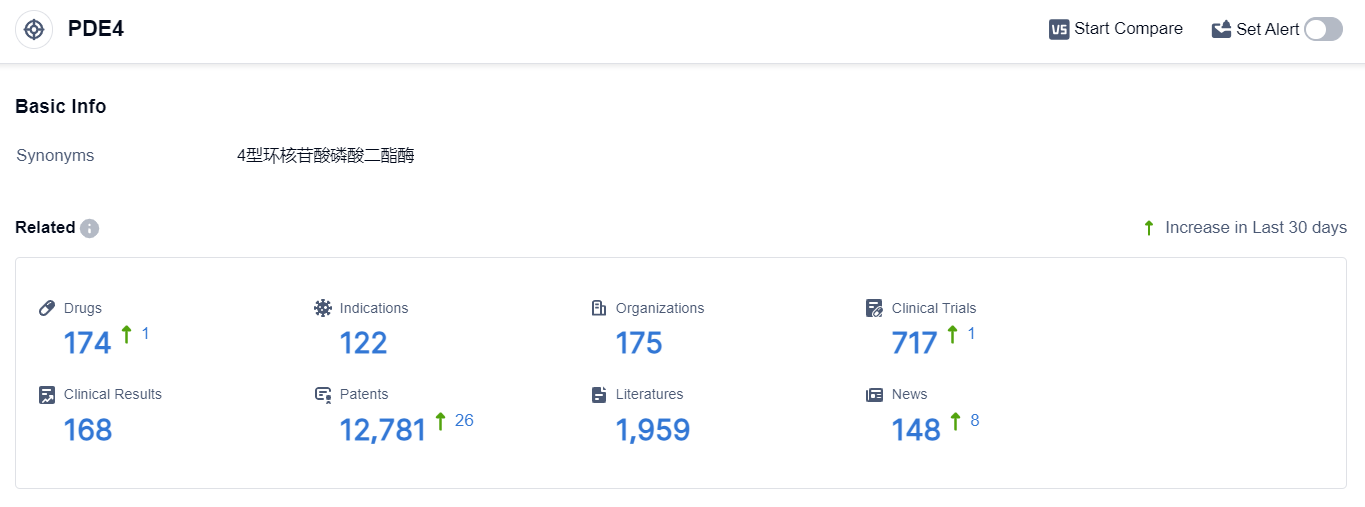
The current competitive landscape of target PDE4 shows that Pfizer Inc. is the leading company with the highest stage of development and a diverse portfolio of drugs. Other companies such as Bristol Myers Squibb Co., Takeda Pharmaceutical Co., Ltd., Arcutis Biotherapeutics, Inc., Amgen, Inc., and Verona Pharma Plc also have drugs in advanced stages of development.
Drugs under the target PDE4 have been approved for various indications, including Plaque psoriasis, Asthma, Pulmonary Disease, Chronic Obstructive, Dermatitis, Atopic, Psoriasis, Behcet Syndrome, Arthritis, Psoriatic, Multiple Sclerosis, and others. This indicates a wide range of therapeutic applications for drugs targeting PDE4.
The most rapidly progressing drug types under the target PDE4 are small molecule drugs and Unknown drugs. The presence of biosimilars is not mentioned in the provided data, so it is not possible to analyze the research and development institutions of these biosimilars.
Countries/locations such as China, United States, European Union, and Japan are developing fastest under the target PDE4, with a significant number of approved drugs and drugs in various stages of development. China, in particular, has shown progress in terms of approved drugs and drugs in Preclinical and Inactive stages.
Overall, the target PDE4 presents a competitive landscape with multiple companies, diverse indications, and different drug types. The future development of target PDE4 will likely involve further research and development in the existing indications and exploration of new therapeutic areas.
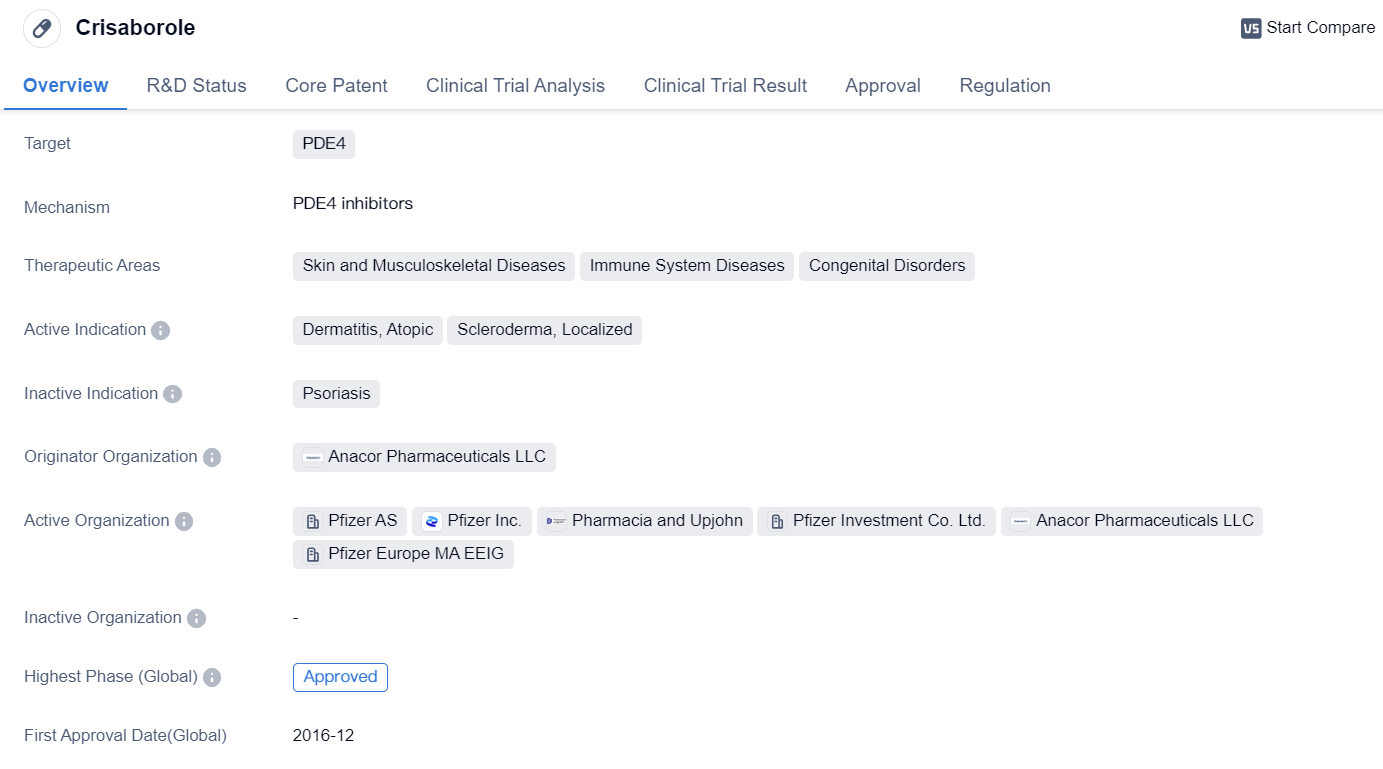
Key Drug: Crisaborole
Crisaborole is a small molecule drug that targets PDE4 and is used in the treatment of various skin and musculoskeletal diseases, immune system diseases, and congenital disorders. The drug has been approved for use in the United States and China.
Crisaborole is primarily indicated for the treatment of dermatitis, atopic, scleroderma, and localized conditions. Dermatitis refers to inflammation of the skin, while atopic dermatitis is a chronic inflammatory skin disease characterized by itchy and inflamed skin. Scleroderma is a group of rare diseases that involve the hardening and tightening of the skin and connective tissues, and localized conditions refer to specific areas of the body affected by skin disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug was developed by Anacor Pharmaceuticals LLC, an originator organization specializing in the pharmaceutical industry. Crisaborole has successfully completed the highest phase of clinical trials and received approval for use in both the United States and China.
Crisaborole was first approved in December 2016 in the United States, making it a relatively recent addition to the market. The drug falls under the category of overseas new drugs urgently needed in clinical settings, indicating its potential significance in addressing unmet medical needs. It also received priority review, suggesting that it may offer significant benefits over existing treatment options.
In summary, Crisaborole is a small molecule drug developed by Anacor Pharmaceuticals LLC. It targets PDE4 and is approved for the treatment of dermatitis, atopic, scleroderma, and localized conditions. The drug has received approval in the United States and China, with its first approval in 2016. Crisaborole falls under the category of overseas new drugs urgently needed in clinical settings and received priority review, indicating its potential significance in addressing unmet medical needs.
Roflumilast
Roflumilast is a small molecule drug that belongs to the class of PDE4 inhibitors. It is primarily used in the treatment of various immune system diseases, congenital disorders, skin and musculoskeletal diseases, and respiratory diseases. The drug has been approved for the treatment of plaque psoriasis, COPD, seborrheic dermatitis, and atopic dermatitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Roflumilast works by inhibiting the enzyme PDE4, which is involved in the regulation of inflammatory responses in the body. By blocking PDE4, the drug helps to reduce inflammation and improve symptoms associated with the aforementioned conditions.
The drug was developed by Takeda Pharmaceutical Co., Ltd., a renowned pharmaceutical company. It received its first approval in the European Union in July 2010, making it available for use in the region. However, its highest phase of development in China is still pending, indicating that it has not yet received approval for use in the country.
Roflumilast has shown promising results in clinical trials and has been proven to be effective in managing the symptoms of plaque psoriasis, COPD, seborrheic dermatitis, and atopic dermatitis. Its approval in the European Union signifies its safety and efficacy profile, allowing healthcare professionals to prescribe it to patients suffering from these conditions.
As a small molecule drug, roflumilast is likely to have a well-defined chemical structure, making it easier to manufacture and administer. This could potentially contribute to its widespread availability and accessibility for patients in need.
In conclusion, roflumilast is a small molecule drug that targets PDE4 and is used in the treatment of various immune system diseases, congenital disorders, skin and musculoskeletal diseases, and respiratory diseases. It has been approved for use in the European Union and has shown efficacy in managing plaque psoriasis, COPD, seborrheic dermatitis, and atopic dermatitis. Its pending status in China suggests that it may soon be available for use in the country. Overall, roflumilast represents a significant advancement in the field of biomedicine and offers potential therapeutic benefits for patients suffering from these conditions.