Abbisko Therapeutics' CSF-1R Inhibitor, Pimicotinib, Approved for Global Multicenter Phase 3 Clinical Trials in Europe
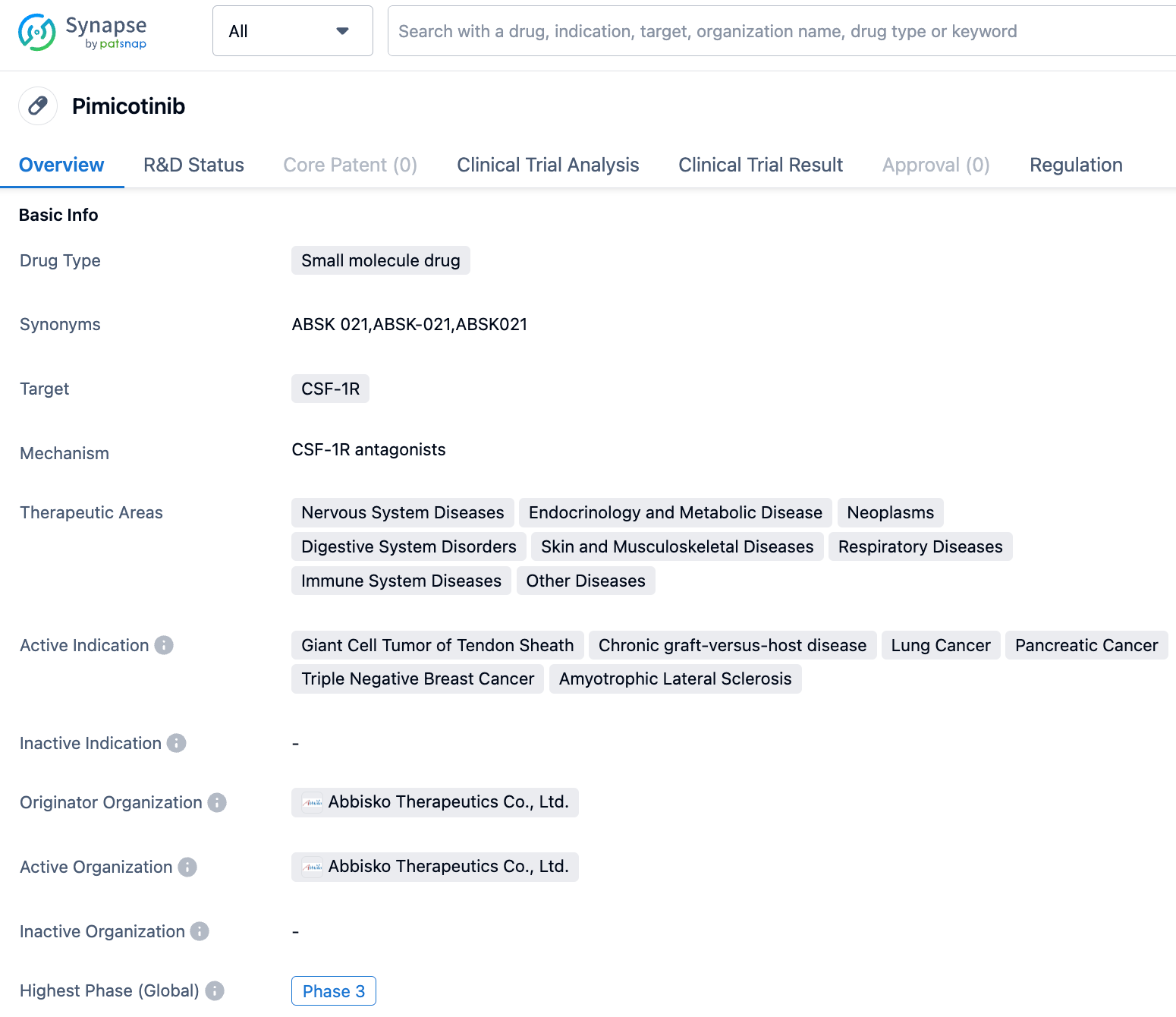
On September 11, 2023, Abbisko Therapeutics announced that its innovative CSF-1R inhibitor pimicotinib (ABSK021) was approved by the European Medicines Agency (EMA) to enter a randomized, double-blind, placebo-controlled, multi-center phase III clinical trial. The study primarily evaluates the drug's effectiveness and safety for patients with tenosynovial giant cell tumor (TGCT).
Pimicotinib is a novel oral, highly selective, highly active small molecule CSF-1R inhibitor developed by Abbisko Therapeutics. Research has shown that blocking the CSF1/CSF-1R signaling pathway can regulate and alter macrophage functions, playing a role in various macrophage-related diseases. Previously, pimicotinib was included in the breakthrough treatment varieties by the Center for Drug Evaluation (CDE) of the China National Medical Products Administration and granted the Breakthrough Therapy Designation by the US FDA. On June 8, 2023, pimicotinib (ABSK021) was granted the PRIME designation by the European EMA for the treatment of inoperable TGCT patients.
The global multi-center phase III clinical study of pimicotinib has been initiated in China and the United States simultaneously and plans to start patient enrolment in Europe soon as well. The results of the study will be used for NDA applications in the Chinese, U.S., and European markets.
The clinical phase 1b trial results of Pimicotinib for the treatment of tenosynovial giant cell tumors were presented at the 2023 American Society of Clinical Oncology (ASCO) meeting. In the study, the candidate drug demonstrated good efficacy and safety. According to the assessment by the Independent Review Committee (IRC) based on RECIST1.1, the objective remission rate of the group receiving a dosage of 50mg once daily reached 77.4%, and no significant hepatic toxicity was observed. 89.8% of patients continue to receive treatment. Joint mobility, pain, and stiffness all showed a trend of alleviation after treatment with Pimicotinib.
CSF-1R is the receptor for the cytokine CSF-1, and is also an important member of the receptor tyrosine kinases, which are closely correlated with the occurrence and development of inflammation. In the tumor microenvironment (TME), the signal of CSF-1R and its ligand CSF-1 can induce the differentiation and survival of tumor-associated macrophages (TAMs), thereby promoting the growth of tumor cells.
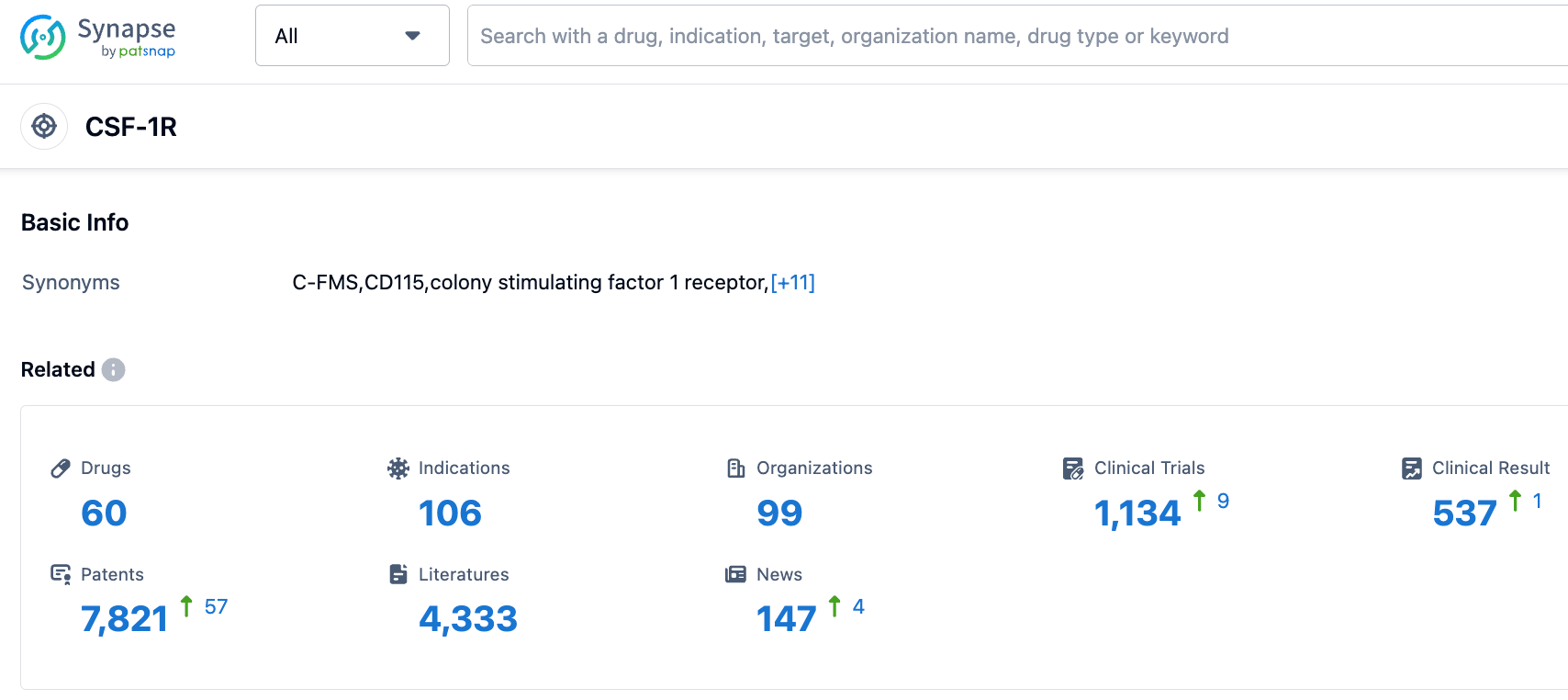
According to the information disclosed by the Synapse Database, as of September 12, 2023, there were a total of 60 drugs under development targeting CSF-1R, covering 106 indications, with 99 institutions engaged in research, involving 1134 related clinical trials, and as many as 7825 patents.
The CSF-1R target has excellent therapeutic potential in anti-tumor treatment, and it is hoped that more such new drugs will be approved for marketing to benefit patients.