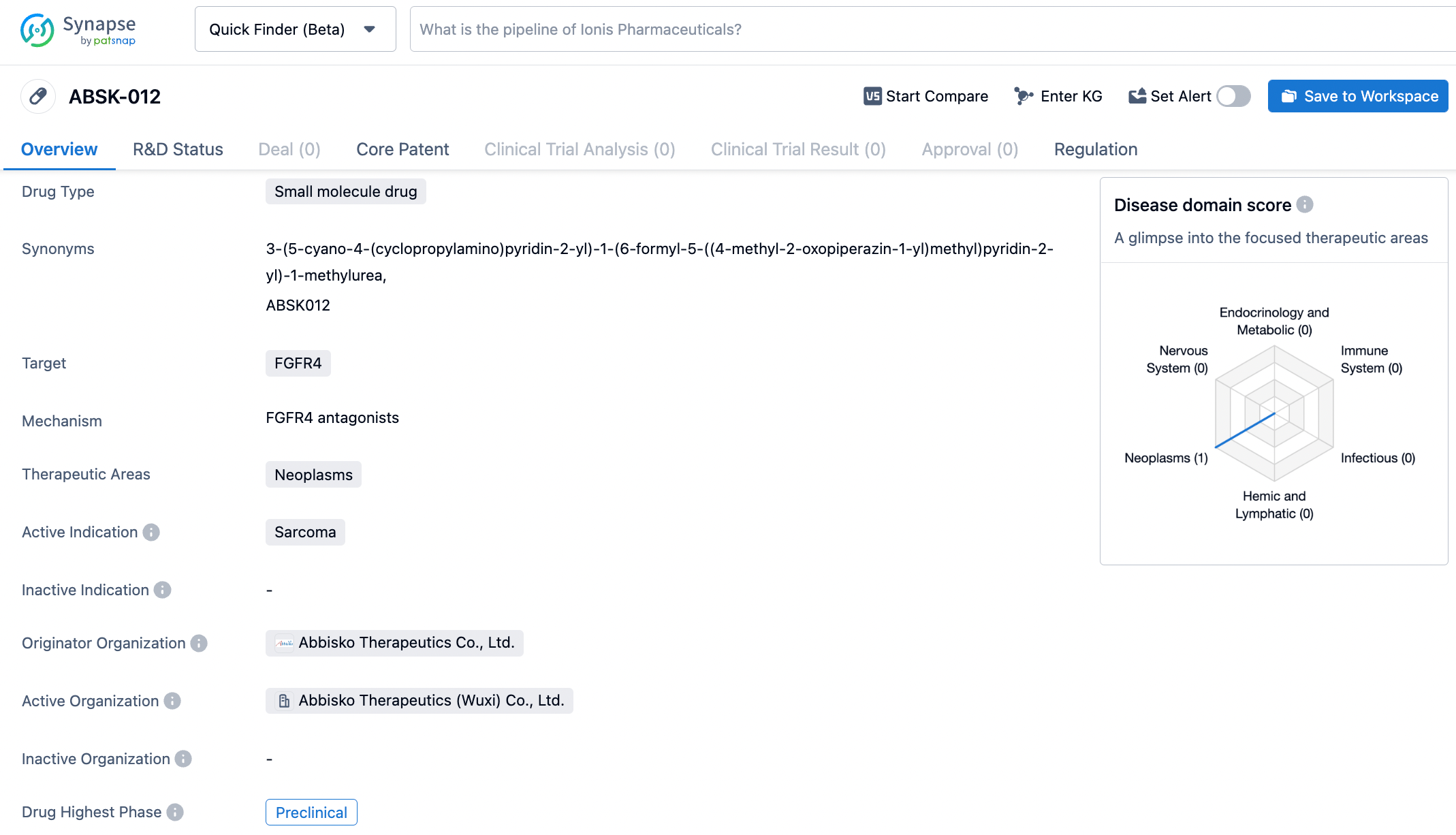
Abbisko Therapeutics' FGFR4 inhibitor, ABSK012, has received FDA clinical trial approval for the treatment of advanced solid tumors
Recently, Abbisko Therapeutics announced that its independently developed anti-drug-resistant mutation small molecule fibroblast growth factor receptor 4 (FGFR4) inhibitor ABSK012 has been approved by the US FDA to commence its first-in-human (FIH) phase I clinical trials in patients with advanced solid tumors.
ABSK012 is a new generation of highly selective small molecule FGFR4 inhibitors that can overcome the FGFR4 drug resistance mutations to the first generation of inhibitors. The FGFR4 signaling pathway is a promising direction for the development of molecular-targeted therapies for solid tumors such as HCC and RMS. In preclinical studies, ABSK012 exhibited anti-tumor activity and has good drug metabolism and pharmacokinetic properties. In April 2023, ABSK012 received Orphan Drug Designation from the US FDA for the treatment of Soft Tissue Sarcoma (STS).
The research that has been approved is an open study evaluating the safety, tolerability, and pharmacokinetics of ABSK012 in patients with advanced solid tumors. The research population includes patients with diseases such as advanced solid tumors with specific genetic changes, FGF19 overexpressing hepatocellular carcinoma (HCC), and FGFR4 mutant rhabdomyosarcoma (RMS).
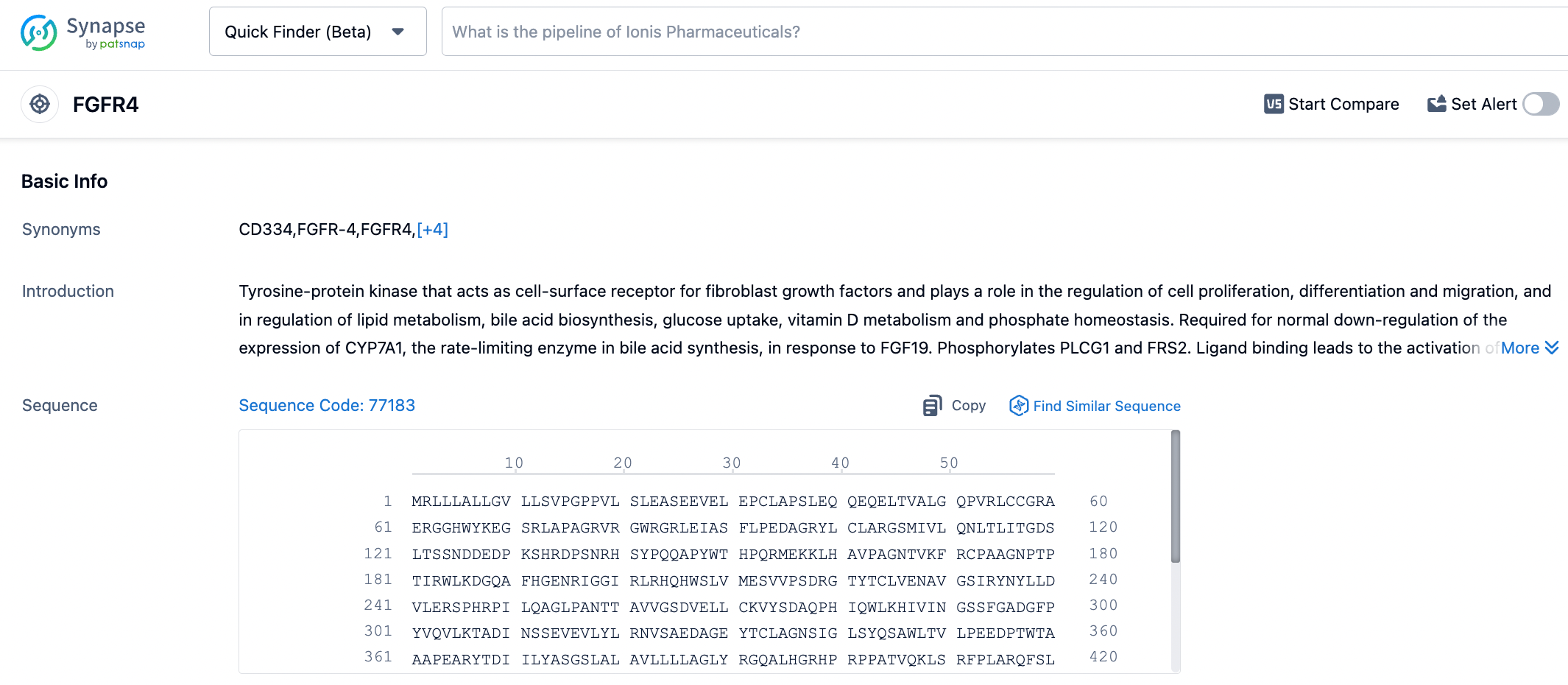
Fibroblast growth factors (FGFs) and their receptors (FGFRs) play important roles in human embryogenesis, angiogenesis, cell proliferation, and differentiation. With the abnormal activation of FGFRs, the FGF/FGFRs signaling pathway is involved in the occurrence and development of almost all malignant tumors. Overexpression of FGF19 and its receptor FGFR4, by activating countless downstream signaling pathways, regulate cell survival, proliferation, invasion, and migration, inducing carcinogenesis. In human liver cancer specimens, aberrant expression of FGF19 and FGFR4 is associated with poor prognosis, and overexpressed FGF19 is involved in liver cancer development. In mouse experiments, overexpressed FGF19 promotes hepatocyte proliferation, leading to poor hepatocyte development and the induction of liver cancer. The use of FGFR4 monoclonal antibodies can inhibit the formation and development of liver cancer in mice overexpressing FGF19.
According to information disclosed in the Synapse database, as of November 9, 2023, there are 45 drug products under research for FGFR4, with 79 indications, 69 research institutions, 706 related clinical trials, and as many as 4244 patents. Frost & Sullivan predicts that the Compound Annual Growth Rate (CAGR) of the Chinese and global FGFR4 drug market between 2025 and 2030 will be 50.9% and 60.1% respectively, and by 2026, the Chinese and global FGFR4 drug market is projected to be $208 million and $555 million respectively, and by 2030 it will be $740 million and $2.435 billion respectively. We look forward to ABSK012 standing out in the FGFR4 target race.