AbbVie Begins Phase 3 Trial for ABBV-383 in Multiple Myeloma, Advancing Oncology Pipeline
AbbVie has reported that the inaugural patient has received treatment with the experimental ABBV-383 in the CERVINO Phase 3 clinical trial. ABBV-383 is a unique bispecific antibody T-cell engager targeting B-cell maturation antigen (BCMA) and CD3, featuring bivalent BCMA-binding domains that offer high avidity for BCMA and a low-affinity CD3 binding domain. Currently, ABBV-383 is being tested in a multicenter, randomized, open-label Phase 3 study, where it is being compared to standard therapies in patients with relapsed or refractory multiple myeloma (r/r MM) who have undergone at least two prior treatments.
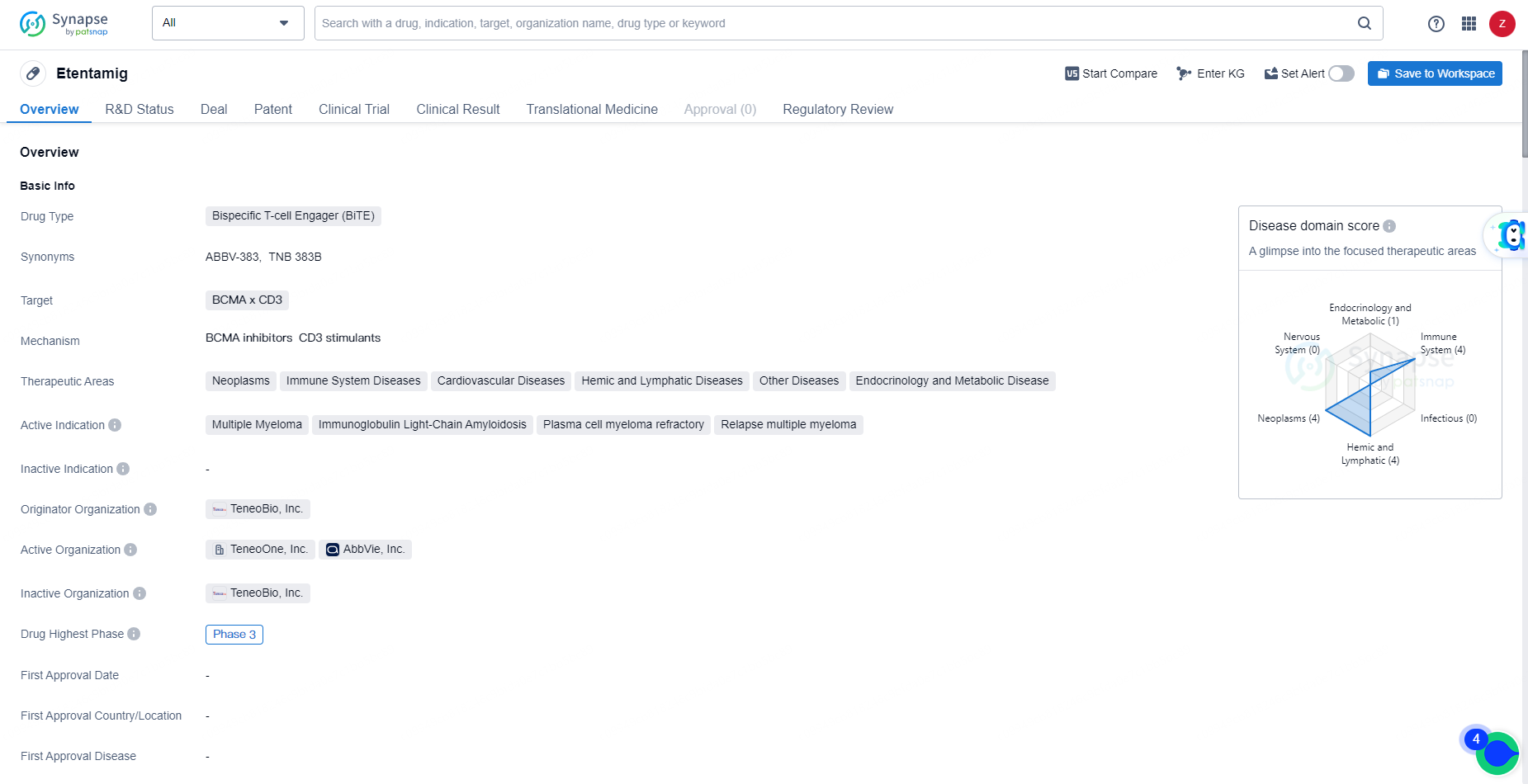
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Even with significant progress in treatment, the majority of patients with multiple myeloma will eventually face relapse. For those with advanced stages of the disease, particularly in community settings, access to new treatment options is often restricted, and existing treatments come with substantial burdens, such as frequent dosing," stated Dr. Peter Voorhees, clinical professor of medicine and director of plasma cell disorders at Atrium Health Levine Cancer Institute. "The CERVINO Phase 3 trial aims to assess the efficacy of ABBV-383 with a monthly dosing schedule, and we are eager to analyze the forthcoming data."
Multiple myeloma, a blood cancer marked by the abnormal growth of plasma cells, can lead to damage in various organs and ranks as the second most common blood cancer globally. Approximately 176,000 people worldwide were diagnosed with multiple myeloma in 2020, with 117,000 deaths resulting from the disease.
"The initiation of the CERVINO Phase 3 trial signifies a significant advancement in AbbVie's ongoing effort to enhance oncology treatments and improve the standard of care for blood cancer patients," remarked Mariana Cota Stirner, M.D., vice president and therapeutic area head of oncology and hematology at AbbVie. "From the start of treatment, ABBV-383 will be evaluated with monthly dosing to potentially simplify the treatment process for both doctors and patients, pending positive trial outcomes."
ABBV-383 is an investigational, unique BCMA x CD3 bispecific antibody T-cell engager, featuring bivalent high-avidity BCMA-binding domains, a low-affinity CD3-binding domain designed to mitigate cytokine release, and a silenced Fc tail engineered for an extended half-life, possibly supporting dosing intervals of every four weeks. The clinical importance of these structure-activity relationships is yet to be determined.
BCMA is predominantly expressed on malignant plasma cells in multiple myeloma, making it an ideal therapeutic target. BCMA is crucial for the survival of myeloma cells, as it fosters their growth and prevents their apoptosis.
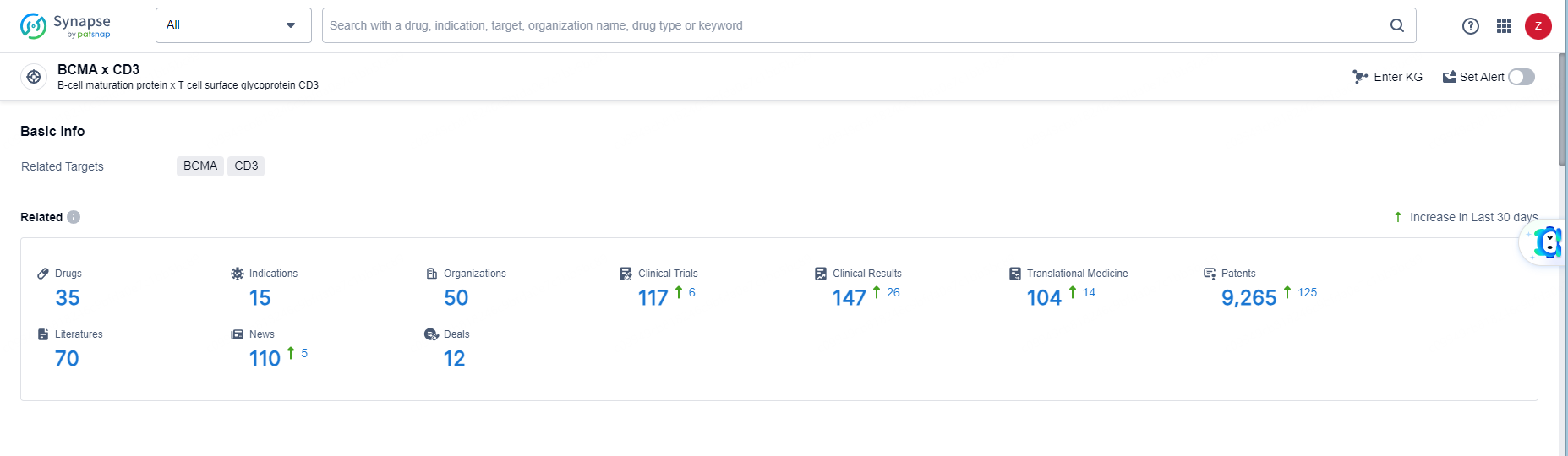
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 11, 2024, there are 35 investigational drugs for the BCMA and CD3 targets, including 15 indications, 50 R&D institutions involved, with related clinical trials reaching 117, and as many as 9265 patents.
Etentamig is intended for the treatment of multiple myeloma, immunoglobulin light-chain amyloidosis, plasma cell myeloma refractory, and relapse multiple myeloma. The drug has reached Phase 3 of development globally and has received IND approval in China.