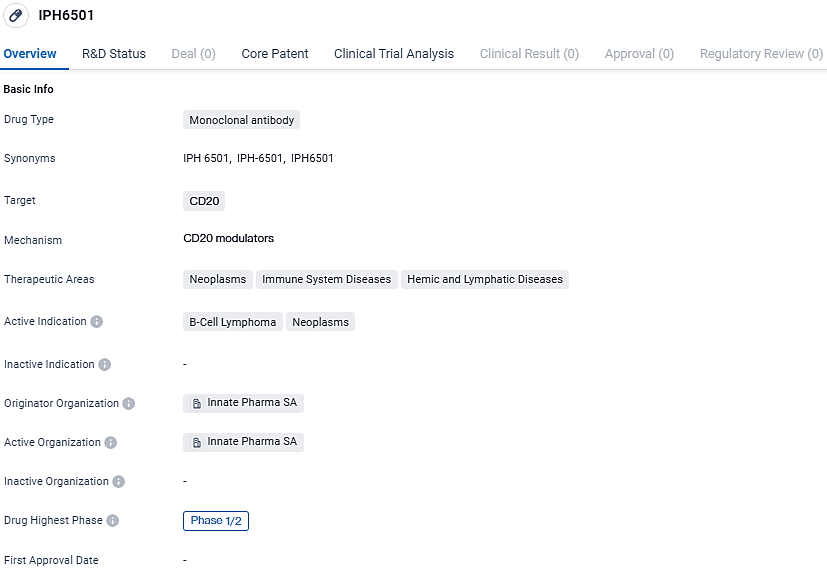
First Patient Treated in IPH6501 Trial for Relapsed/Refractory B-Cell NHL
Innate Pharma SA has publicized the commencement of their Phase 1/2 clinical study across various centers, with the initial participant receiving a dose of the investigational medicine IPH6501. This trial seeks to assess the drug's safety profiles and how well patients can endure it, specifically targeting individuals diagnosed with B-cell Non-Hodgkin’s Lymphoma expressing the CD20 marker who have experienced a relapse or have shown resistance to prior treatments.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
IPH6501 represents Innate Pharma's pioneering CD20-directed tetraspecific ANKET® therapy, designed to selectively bind to CD20 present on harmful B cells, while also interacting with three distinct receptors on NK cells: a pair of stimulating receptors and the interleukin-2 receptor, complemented by a modified version of human IL-2. This innovative approach stimulates NK cell expansion and augments their innate ability to target and destroy CD20 positive malignancies. The clinical trial aims to include up to 184 participants.
"We're excited to report that a first participant has begun treatment in the Phase 1/2 trial exploring IPH6501, our unique ANKET® compound, marking the first time a tetraspecific NK Cell Engager has been tested in a clinical setting," remarked Dr Sonia Quaratino, Innate Pharma's Chief Medical Officer.
"Our tetraspecific ANKET® molecules, now with the added IL-2 variant, are equipped to intensify the proliferative and functional power of NK cells in combating tumor cells. IPH6501 is therefore a significant advance and provides an alternative to the T cell-based approaches in treating B-cell non-Hodgkin's lymphomas," further commented Dr Quaratino.
"Recent advancements in chemotherapy, T-cell immunotherapy, and targeted treatments have vastly improved the prognosis for patients suffering from B-cell non-Hodgkin's lymphomas, making them superior to the outcomes of conventional chemotherapy," Dr Lorenzo Falchi, a Lymphoma Expert at the Memorial Sloan Kettering Cancer Center in New York, and the lead investigator for this study, added to the conversation.
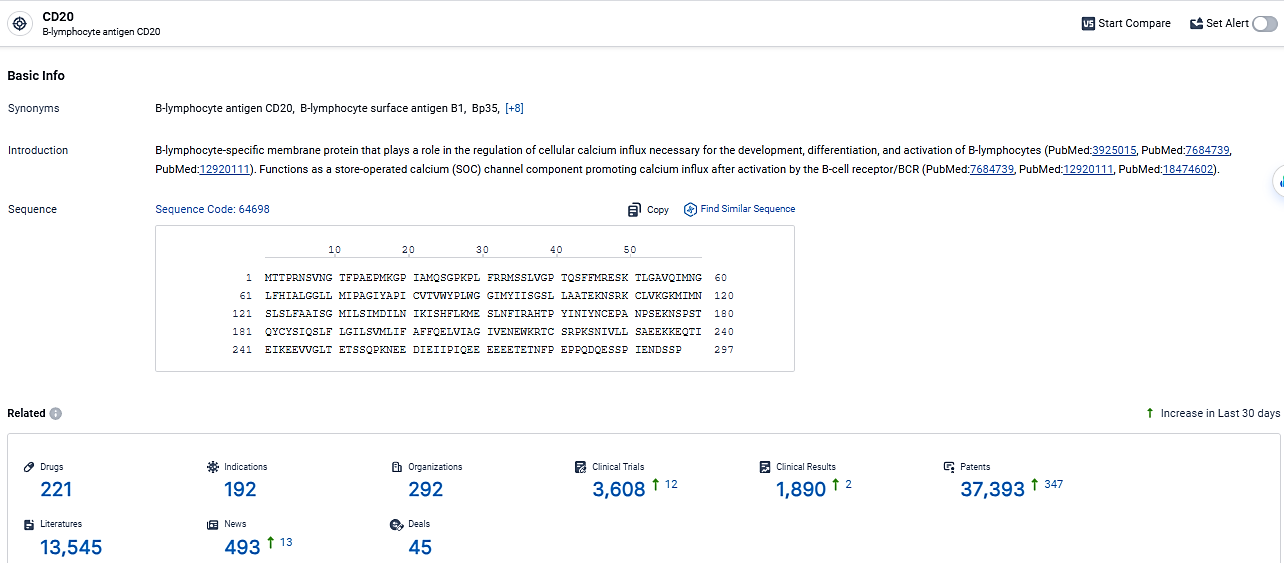
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 6, 2024, there are 221 investigational drugs for the CD20 target, including 192 indications,292 R&D institutions involved, with related clinical trials reaching 3608, and as many as 37393 patents.
IPH6501 shows promise as a potential treatment for B-cell lymphoma and other neoplasms. Its specific targeting of CD20 and its current phase of development indicate that it has already shown some positive results in early clinical trials. Further research and testing will be needed to determine its safety and efficacy in larger patient populations.