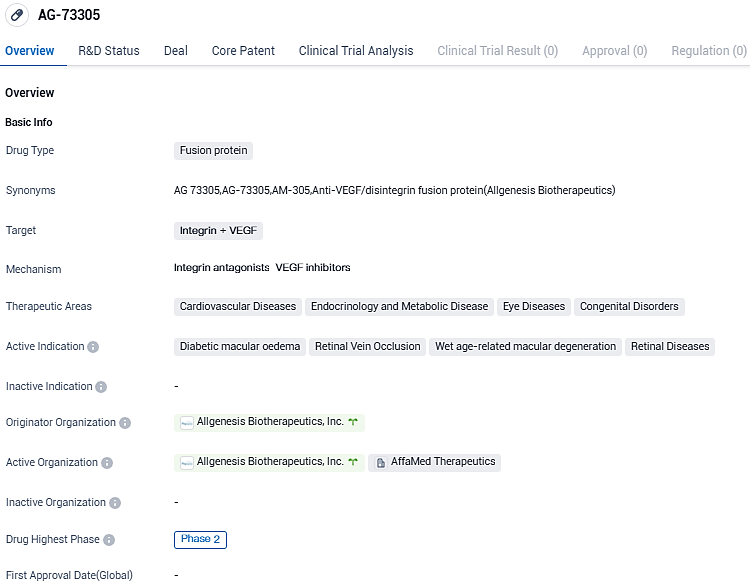
Allgenesis announces positive early results for its AG-73305 phase 2a Diabetic Macular Edema study at the American Academy of Ophthalmology
At the 2023 American Academy of Ophthalmology conference that took place from November 2 to 6 in San Francisco, Allgenesis Biotherapeutics Inc. revealed encouraging initial results from the debut human trial, phase 2a, evaluating the safeness, acceptability, and potency of AG-73305 in treating Diabetic Macular Edema patients. The evidence was introduced during a paper presentation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Initial findings from the trial involving 22 patients indicated that AG-73305, an Fc-fusion protein designed to inhibit VEGF and integrin pathways, was safe and well-tolerated following a single intravitreal injection. No dose-related toxicities or serious adverse events (SAEs) connected to AG-73305 were observed in the patients.
The mean improvement in BCVA was +6.4 ETDRS letters among the four cohorts, and there was a decrease of -100 microns in CST four weeks post-injection. The impact lasted for 12 to 24 weeks after a one-time injection, with around 66% chance shown by the Kaplan-Meier analysis that patients didn't need rescue by the 24th week.
Madhu Cherukury, Ph.D., DABT., CEO of Allgenesis, expressed excitement about revealing this initial data at AAO and about the positive reaction it elicited from the ophthalmology community. "Our Phase 2a study data supports the supposition that inhibiting multiple pathways in the disease can offer additional advantages to patients with DME in the form of BCVA gains, with the possibility to strive for extended durability versus the current standard care," Cherukury said.
Dr. Cherukury further stated, "Given the promising data from the open label trial, we are vigorously proceeding with our development plans' consecutive phases and anticipate revealing AG73305's full potential in a Phase 2b trial involving DME patients."
In September 2021, Allgenesis declared a licensing agreement with AffaMed Therapeutics for the AG-73305's development and commercialization in Greater China, South Korea, and numerous ASEAN markets.
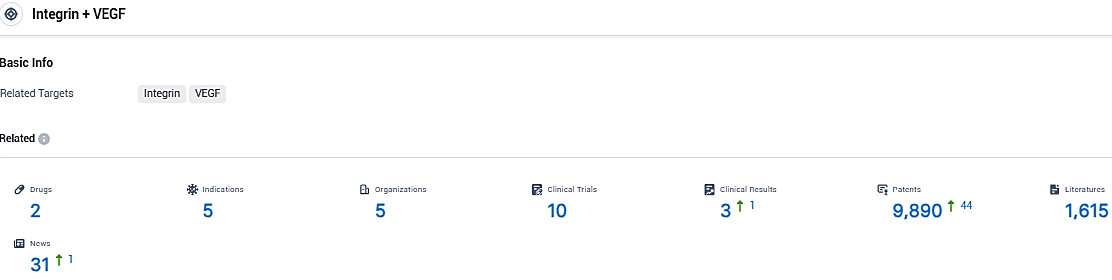
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 16, 2023, there are 2 investigational drugs for the VEGF and integrin target, including 5159 indications, 5 R&D institutions involved, with related clinical trials reaching 10, and as many as 9890 patents.
AG-73305 is a humanized, bi-specific Fc-fusion protein designed to simultaneously block VEGFs and integrins for the treatment of DME, nAMD, RVO, and other retinal diseases. AG-73305 contains a VEGF-trap and a disintegrin that blocks various key integrin receptors. AG-73305 has the potential to treat both anti-VEGF responders and non-responders. The Phase 2a first-in-human study is expected to be completed in March 2024.