AstraZeneca's Novel GR/β2AR Targeted Therapy for Asthma
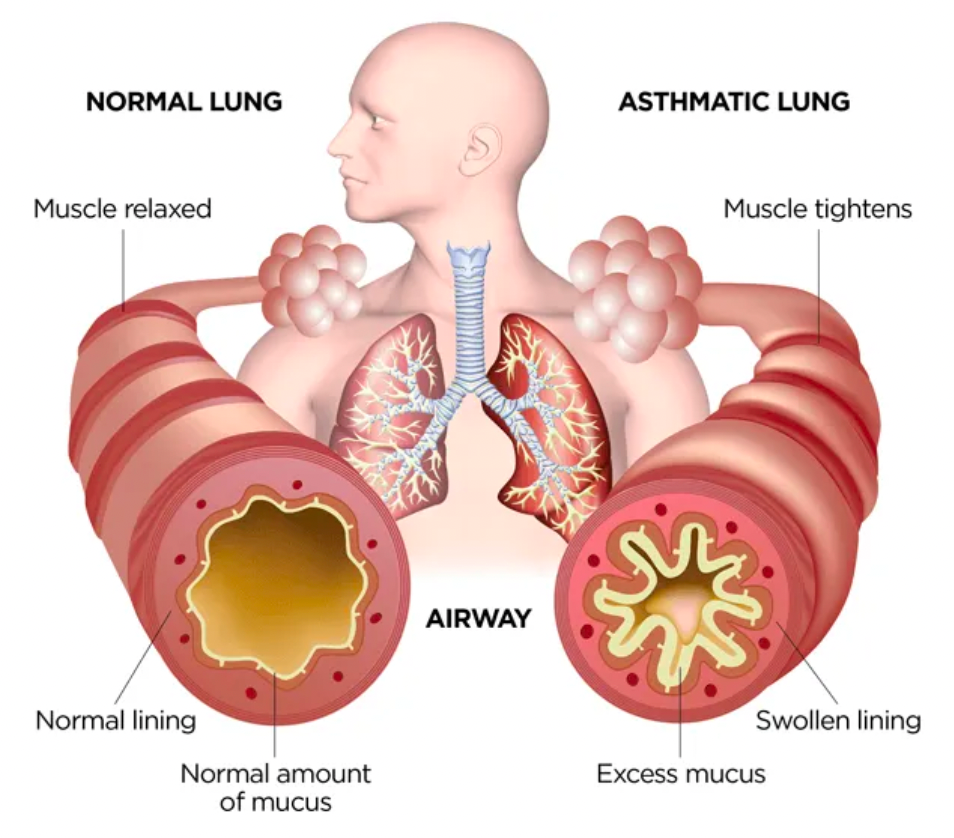
Asthma is a chronic respiratory disease characterized by chronic inflammation of the airways and reversible airway obstruction. Individuals with asthma have sensitive airways that can narrow when exposed to triggers, leading to difficulties in breathing, wheezing, coughing, and a feeling of chest tightness. Asthma patients experience recurrent episodes of breathing difficulties and wheezing that vary in severity and frequency over time.
Globally, as many as 262 million people are affected by asthma, with about 25 million in the United States alone. These patients face the risk of severe exacerbations regardless of the severity of their condition, adherence to treatment, or level of control. It is estimated that there are 136 million exacerbations of asthma annually worldwide. These exacerbations pose a physical threat and emotional impact on many patients and can be fatal.
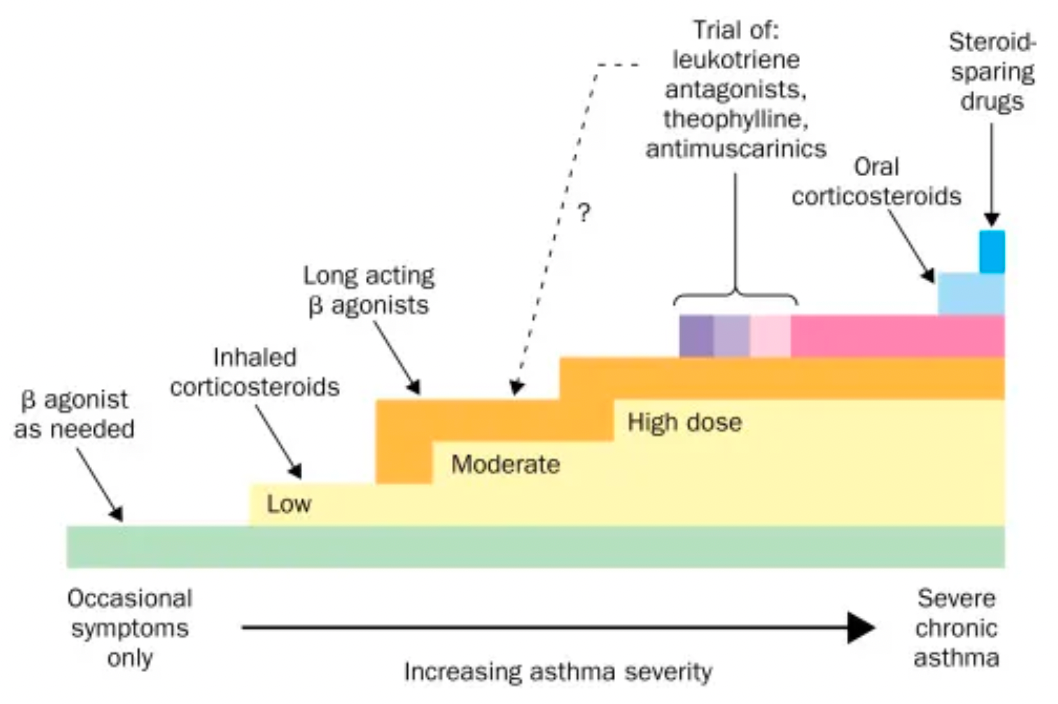
Many patients with asthma symptoms use short-acting β2-adrenergic receptor agonists, such as albuterol, as rescue medications. However, the use of short-acting β2-adrenergic receptor agonists alone does not address the underlying inflammation, leaving patients at risk of severe exacerbations which can lead to decreased quality of life, hospitalization, and frequent use of oral corticosteroids. Additionally, risks of adverse health outcomes are associated with 1-3 short courses of oral corticosteroid treatment of exacerbation, including type 2 diabetes, depression/anxiety, renal impairment, cataracts, cardiovascular disease, pneumonia, and fractures.
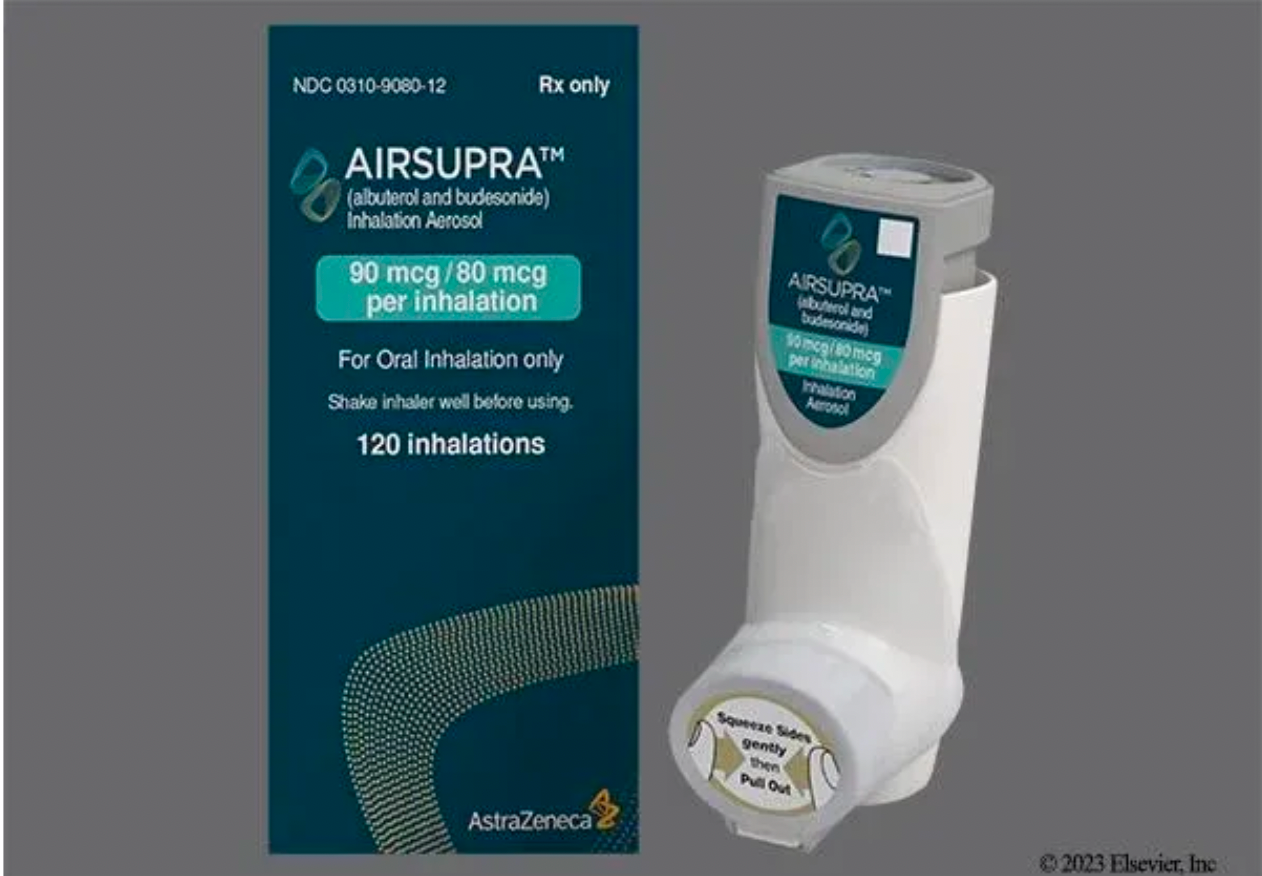
In January 2023, AstraZeneca's official website announced that Airsupra (albuterol/budesonide, PT027), the first and only rescue medication approved in the United States for as-needed use to reduce the risk of asthma exacerbation, has been authorized in the U.S. for as-needed treatment or prevention of bronchospasm, and to lower the risk of asthma exacerbation in patients aged 18 years and older.

The approved Airsupra is a pressurized metered-dose inhaler (pMDI) and fixed-dose combination rescue medication, co-developed by AstraZeneca and Avillion, containing the short-acting β2-adrenergic agonist albuterol and the anti-inflammatory inhaled corticosteroid budesonide.
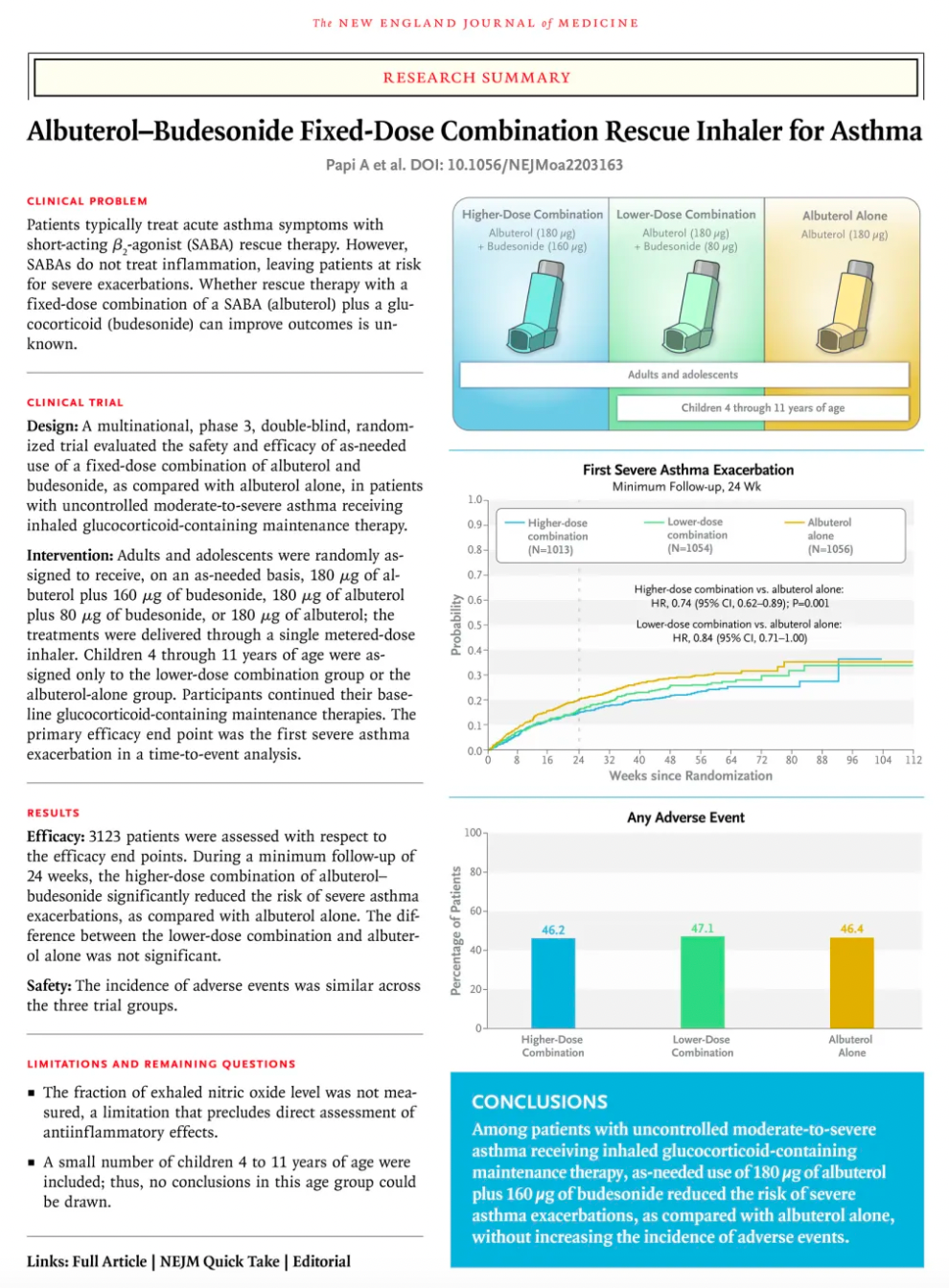
Based on the results of a clinical study (MANDALA trial) published in the New England Journal of Medicine in May 2022, the combination therapy of Airsupra was shown to significantly reduce the risk of severe exacerbations in patients with moderate to severe asthma when used as-needed as a rescue medication, compared to the use of albuterol alone. Importantly, at the approved dose (90 mcg albuterol/80 mcg budesonide), Airsupra significantly reduced the average annualized total systemic corticosteroid exposure, a key secondary endpoint, compared to albuterol.
According to statistics from the Synapse database, there are 1,565 investigational drugs and 148 marketed drugs globally for the treatment of asthma. The β2-adrenergic receptors and glucocorticoid receptors mentioned in this article are the most important therapeutic targets for asthma. In addition, other popular targets include the H1 histamine receptor, acetylcholine receptor, IgE, CysLT, IL-5, etc. Among them, monoclonal antibody drugs targeting IgE (Omalizumab), IL-5 (Mepolizumab, Reslizumab), and TSLP (Tezepelumab) have been approved and launched on the market in recent years.
β2AR and Compound Asthma Medication
The β2 adrenergic receptor (β2AR) is one of the most classic members of the GPCR family. Due to its extensive distribution in lung tissues, it plays a critical role in the pathogenesis and treatment of asthma. Activation of β2 adrenergic receptors results in an increase in intracellular cAMP levels, which in turn activates protein kinase A. The activation of protein kinase A leads to relaxation of smooth muscles, particularly the relaxation of bronchial smooth muscles. This effect aids in the dilation of airways, reduction of respiratory resistance, and improvement of ventilation capacity.
In asthma patients, the bronchial smooth muscles are more sensitive to various stimuli, causing airway constriction. Activation of β2 adrenergic receptors helps to mitigate this airway constriction and improve breathing by promoting the relaxation of the bronchial smooth muscles. Therefore, β2 adrenergic receptor agonists are commonly used medications for the treatment of asthma.
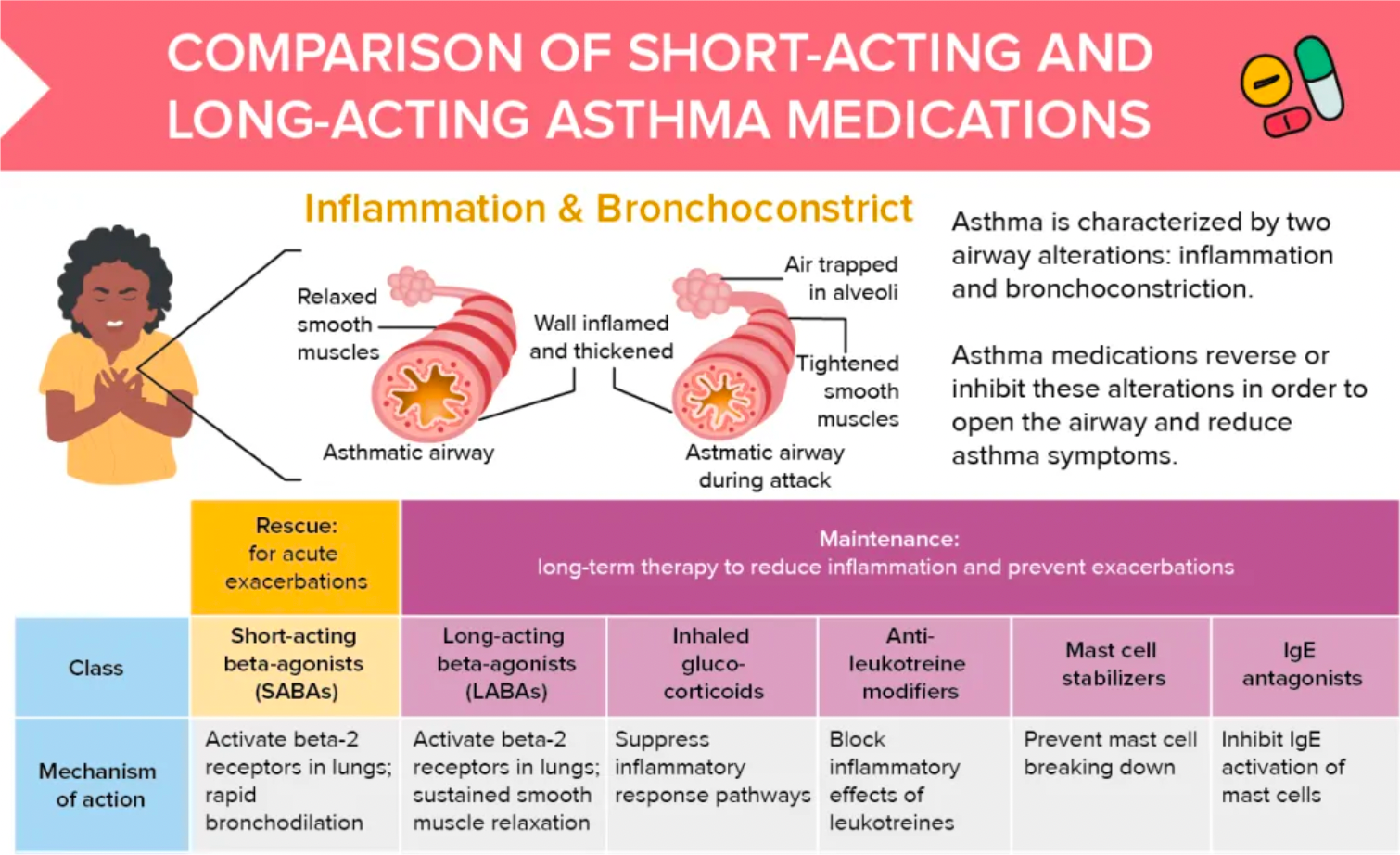
β2 agonists are divided into two categories: short-acting and long-acting. Short-acting β2 agonists are primarily used for emergency relief to quickly alleviate acute asthma symptoms. Long-acting β2 agonists are usually used for the long-term control of asthma and can help prevent the occurrence of symptoms.
More than half of the drugs that target β2 adrenergic receptors worldwide, either in clinical research or approved for clinical use, are related to the treatment of asthma. This includes asthma, chronic obstructive pulmonary disease (COPD), mild asthma, allergic asthma, and more.
In addition to the development of non-selective or selective agonists targeting β2 adrenergic receptors alone, β2 adrenergic receptors are often paired with other asthma targets for the development of combination formulations. Common combinations include those with GR (glucocorticoid receptors), mAChRs (muscarinic acetylcholine receptors), H1R (histamine receptor 1), β1AR (beta-1 adrenergic receptors), DRDs (dopamine receptors), and others.
Combination asthma medications are designed to control asthma symptoms more comprehensively through different mechanisms of action. Compared to single-component β2 agonists, combination asthma medications better meet patients' needs and offer a more comprehensive treatment effect.
Inhaled corticosteroids: Inhaled corticosteroids (such as fluticasone, budesonide, etc.) are commonly used drugs for controlling asthma inflammation. They can reduce the inflammatory response within the airways and decrease mucus production, thereby helping to alleviate airway obstruction.
Leukotriene modifiers: For example, montelukast is a leukotriene modifier that can block the actions of leukotrienes, reducing the inflammatory response and lowering airway hyperresponsiveness.
Some combination asthma medications combine these different types of drugs to simultaneously relieve asthma symptoms through multiple pathways. Such combinations can enhance therapeutic efficacy to some extent, reduce the frequency of medication use, and improve patient compliance.