FDA Approves VOYDEYA™ for Extravascular Hemolysis in PNH with Ravulizumab or Eculizumab
FDA has granted authorization for the utilization of VOYDEYA™ (danicopan) as a supplementary treatment in conjunction with ravulizumab or eculizumab. This is specifically for managing adult patients experiencing extravascular hemolysis due to paroxysmal nocturnal hemoglobinuria.
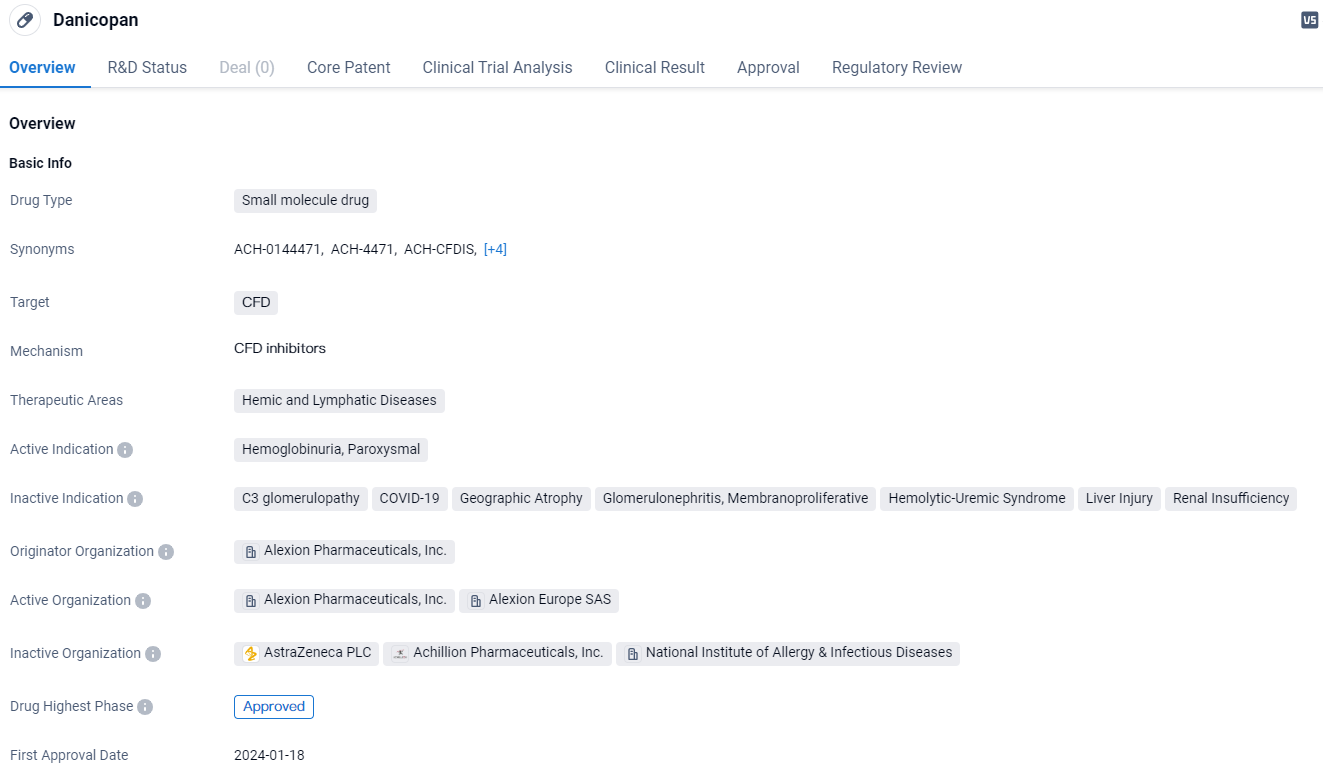
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
VOYDEYA represents a groundbreaking, orally administered Factor D inhibitor, created to complement ULTOMIRIS (ravulizumab-cwvz) or SOLIRIS (eculizumab), the existing standard treatments for paroxysmal nocturnal hemoglobinuria (PNH). This new medication is intended to benefit the 10-20% portion of PNH patients who still suffer from severe extravascular hemolysis (EVH) even while receiving C5 inhibitor therapies.
The authorization of VOYDEYA by the US Food and Drug Administration (FDA) stems from encouraging outcomes derived from the crucial ALPHA Phase III study. Highlights of this 12-week study's primary assessment phase were featured in a publication by The Lancet Haematology.
Alexion's CEO, Marc Dunoyer, remarked on the significance of VOYDEYA's sanctioning, “Introducing the pioneering Factor D inhibitor VOYDEYA signifies a major leap in PNH management and solidifies our role at the forefront of innovation within complement therapeutics. The data from the ALPHA study indicates that concurrently targeting both Factor D and C5 complements pathways could deliver a superior treatment strategy for PNH patients dealing with EVH, while still maintaining trusted standard therapies.”
The ALPHA Phase III investigation sought to determine the efficacy and safety profile of VOYDEYA used alongside either ULTOMIRIS or SOLIRIS in PNH patients enduring severe EVH. Findings revealed that VOYDEYA achieved the leading goal, which was a hemoglobin level increase from the initial baseline to week 12, in addition to satisfying all major secondary objectives, such as steering clear of blood transfusions and improving scores on the Functional Assessment of Chronic Illness Therapy – Fatigue.
Further insights from the ALPHA Phase III study indicated that VOYDEYA generally had good tolerability among patients, with no emerging safety issues being flagged. Common adverse events occurring during treatment included headache, nausea, joint pain, and diarrhea.
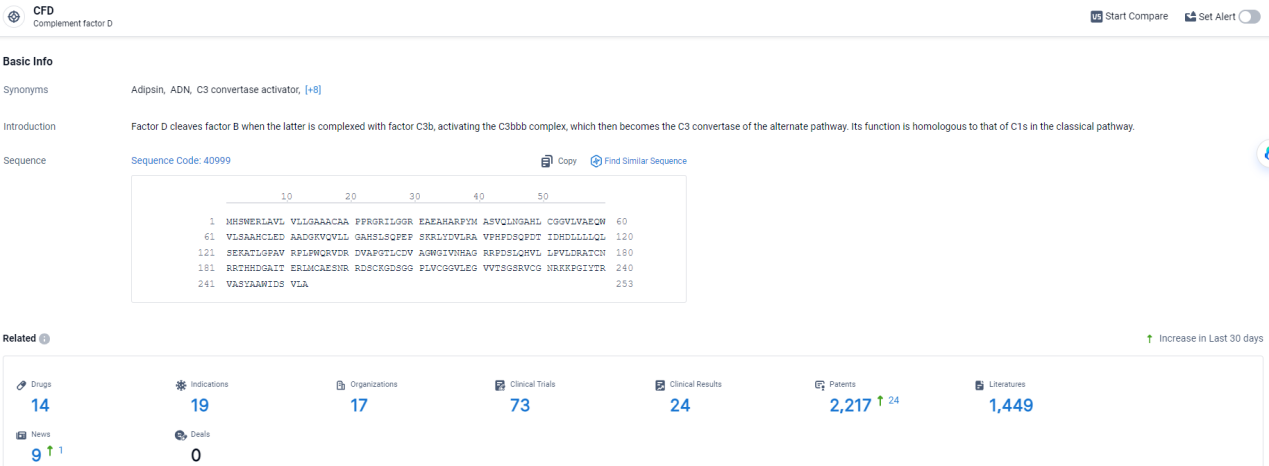
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 3, 2024, there are 14 investigational drugs for the CFD target, including 19 indications, 17 R&D institutions involved, with related clinical trials reaching 73, and as many as 2217 patents.
Danicopan targets CFD and has been approved for the treatment of Hemoglobinuria, Paroxysmal. The drug received its first approval in Japan in 2024 and has reached the highest phase of development. It has also been granted several regulatory designations and plans, including the Paediatric investigation plan, PRIME, Breakthrough Therapy, and Orphan Drug status. These designations aim to facilitate the drug's development and availability, particularly for patients with unmet medical needs.