Adicet Bio Receives FDA Approval for IND Application of ADI-270 in Kidney Cancer
Adicet Bio, Inc., a biotechnology firm in the clinical stage focusing on creating and advancing allogeneic gamma delta T cell therapies for cancer and autoimmune disorders, has reported that the U.S. Food and Drug Administration has approved their Investigational New Drug (IND) application. This IND will enable the assessment of ADI-270, an armored allogeneic "off-the-shelf" gamma delta chimeric antigen receptor (CAR) T cell therapy targeting CD70+ malignancies, for the therapeutic intervention in relapsed/refractory renal cell carcinoma.
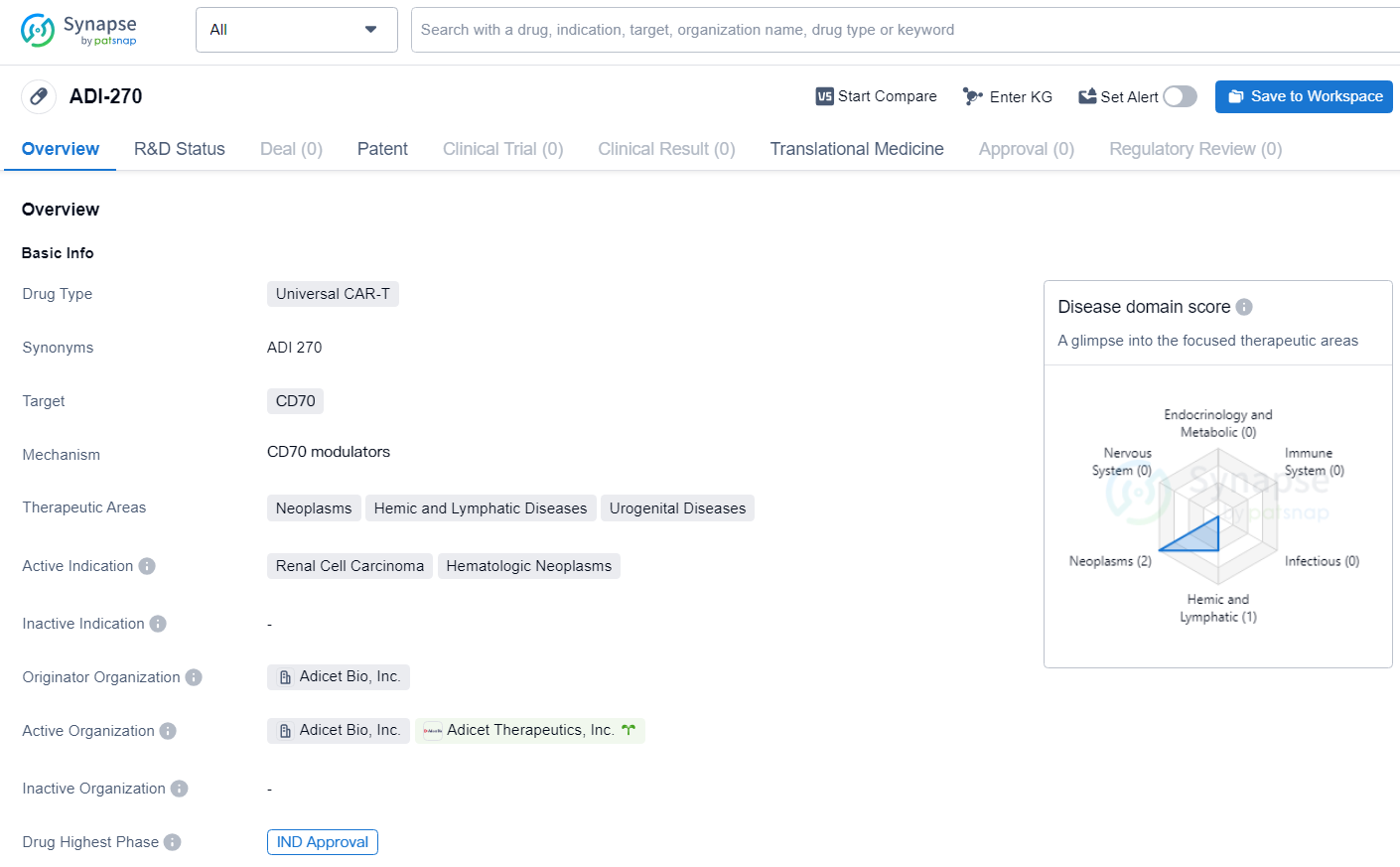
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Chen Schor, the President and CEO of Adicet Bio, stated, "The FDA’s approval of our IND application to study ADI-270 in patients with RCC marks a pivotal milestone for Adicet. ADI-270 is a third-generation CAR T therapy specifically designed to target CD70+ tumors with high accuracy, enhanced exposure, longevity, and tumor infiltration, while mitigating immunosuppressive factors within the tumor microenvironment."
The Phase 1 clinical trial, which is multicenter and open-label, aims to evaluate ADI-270 as a monotherapy in adults with relapsed or refractory clear cell RCC. After lymphodepletion, participants will be eligible to receive a single dose of ADI-270, starting at a dose level of 3E8 CAR+ cells. Depending on protocol criteria, participants who meet the requirements may qualify for a second dose.
The dose escalation and dose expansion phases of the trial will assess the safety, tolerability, and pharmacokinetics of ADI-270, as well as its anti-tumor activity, measured by overall response rate, duration of response, and disease control rate.
ADI-270 is an advanced armored allogeneic gamma delta CAR T cell therapy candidate, developed as an "off-the-shelf" product targeting CD70-positive cancers. CD70 is a prime target due to its significant expression in both solid and hematological malignancies. ADI-270 utilizes a third-generation CAR design to target CD70 through its natural receptor, CD27, and is armored with a dominant negative Transforming growth factor-β receptor II (dnTGFβRII) to counteract the immunosuppressive tumor microenvironment.
Additionally, ADI-270 is engineered to increase exposure and longevity by decreasing its vulnerability to host versus graft rejection. These properties, combined with the strong tumor infiltration capabilities of gamma delta 1 T cells, aim to enhance the clinical outcomes for RCC patients and others with CD70+ tumors.
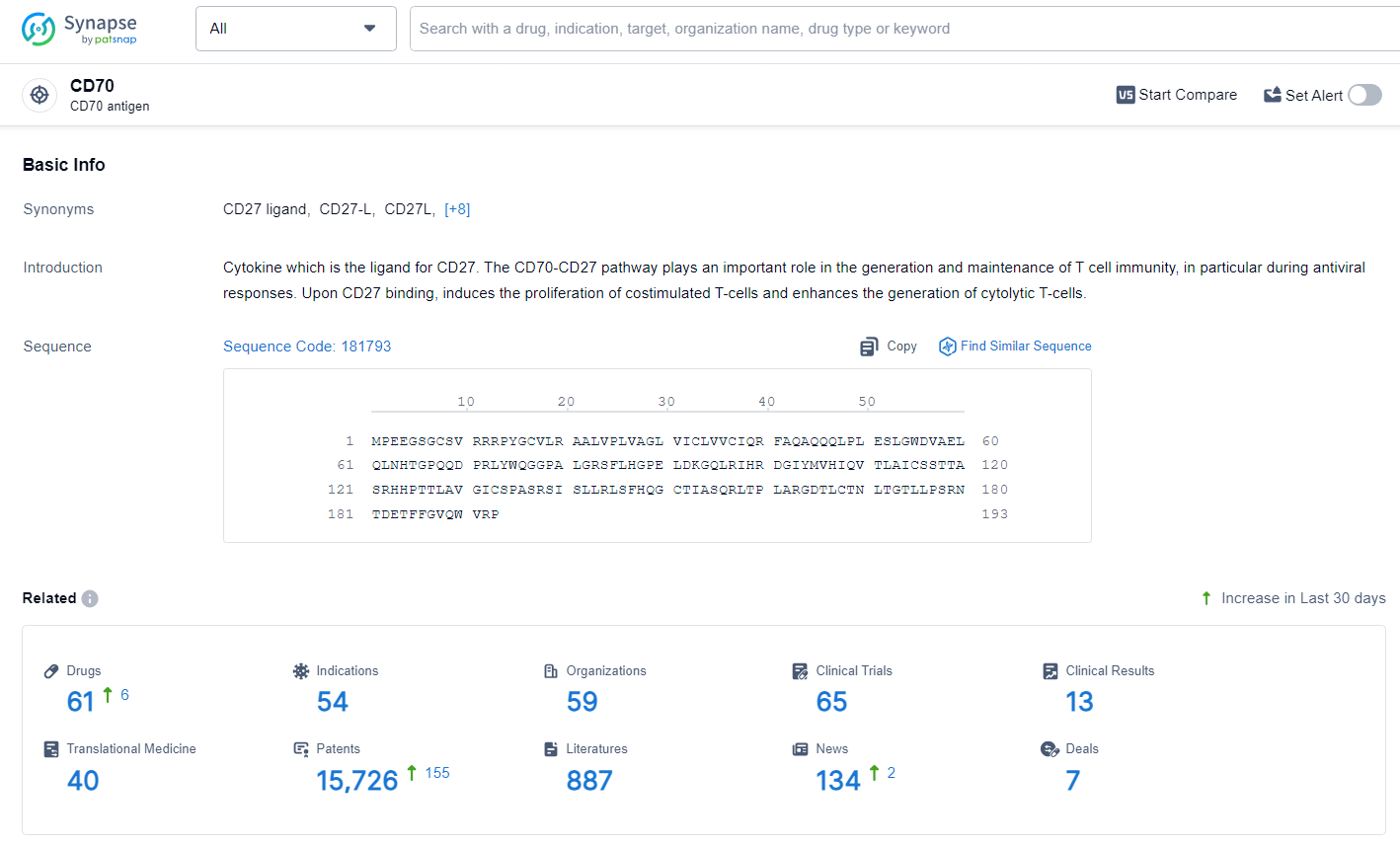
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 61 investigational drugs for the CD70 target, including 54 indications, 59 R&D institutions involved, with related clinical trials reaching 65, and as many as 15726 patents.
ADI-270 is a Universal CAR-T drug with a focus on targeting CD70 for the treatment of various cancers and blood-related diseases. Its current active indications include renal cell carcinoma and hematologic neoplasms, and it has received IND approval, signifying its progression to clinical trials. As the drug continues to advance through the development process, it holds promise for potentially addressing unmet medical needs in the field of biomedicine.