Is Rimegepant approved by the FDA?
Yes, Rimegepant is FDA approved. The U.S. Food and Drug Administration (FDA) approved Rimegepant, under the brand name Nurtec ODT, on February 27, 2020. This medication belongs to the drug class of CGRP (calcitonin gene-related peptide) inhibitors.
What is Rimegepant?
Rimegepant is a prescription medication used for the acute treatment of migraines, with or without aura, in adults. Additionally, it is used to prevent episodic migraines. This medication helps alleviate symptoms such as headache, nausea, vomiting, and sensitivity to light and sound by blocking CGRP receptors.
How Does Rimegepant Work?
Rimegepant works by targeting and blocking the CGRP receptors, which play a significant role in migraine pathophysiology. By inhibiting these receptors, Rimegepant helps reduce migraine symptoms and, when taken regularly, can decrease the frequency of migraine attacks.
Dosage and Administration
Rimegepant is administered as an orally disintegrating tablet (ODT), which dissolves in the mouth without the need for water. The typical dosage is 75 mg, and the tablet should be taken as follows:
- Place the tablet on or under the tongue and allow it to disintegrate in saliva before swallowing.
- Do not take more than one tablet in a 24-hour period.
Usage Guidelines
- Acute Treatment: Take one tablet as soon as migraine symptoms appear.
- Preventive Treatment: Regular use can help reduce the number of migraine days each month.
Warnings and Precautions
- Frequency: Do not exceed treating more than 15 migraines in a 30-day period.
- Allergies: Avoid use if allergic to Rimegepant.
- Medical Conditions: Inform your healthcare provider if you have liver or kidney disease.
- Pregnancy and Breastfeeding: The safety of Rimegepant during pregnancy and breastfeeding is not well-established. Consult your doctor for advice.
Side Effects
Common side effects of Rimegepant include:
- Nausea
- Allergic reactions (hives, rash, difficulty breathing, swelling of the face, lips, tongue, or throat)
Drug Interactions
Rimegepant can interact with other medications, affecting its efficacy and increasing side effects. Avoid taking Rimegepant within 48 hours of using the following:
- Aprepitant
- Erythromycin
- Fluconazole
- Heart or blood pressure medications like diltiazem and verapamil
Storage
Store Rimegepant at room temperature, away from moisture and heat. Keep it in its original blister pack until ready for use.
Conclusion
Rimegepant (Nurtec ODT) is an FDA-approved medication for the acute treatment and prevention of migraines in adults. Approved on February 27, 2020, it provides a quick-dissolving, effective option for managing migraine symptoms and reducing the frequency of episodic migraines. Always consult with a healthcare provider for personalized advice and follow all prescribed guidelines for safe and effective use.
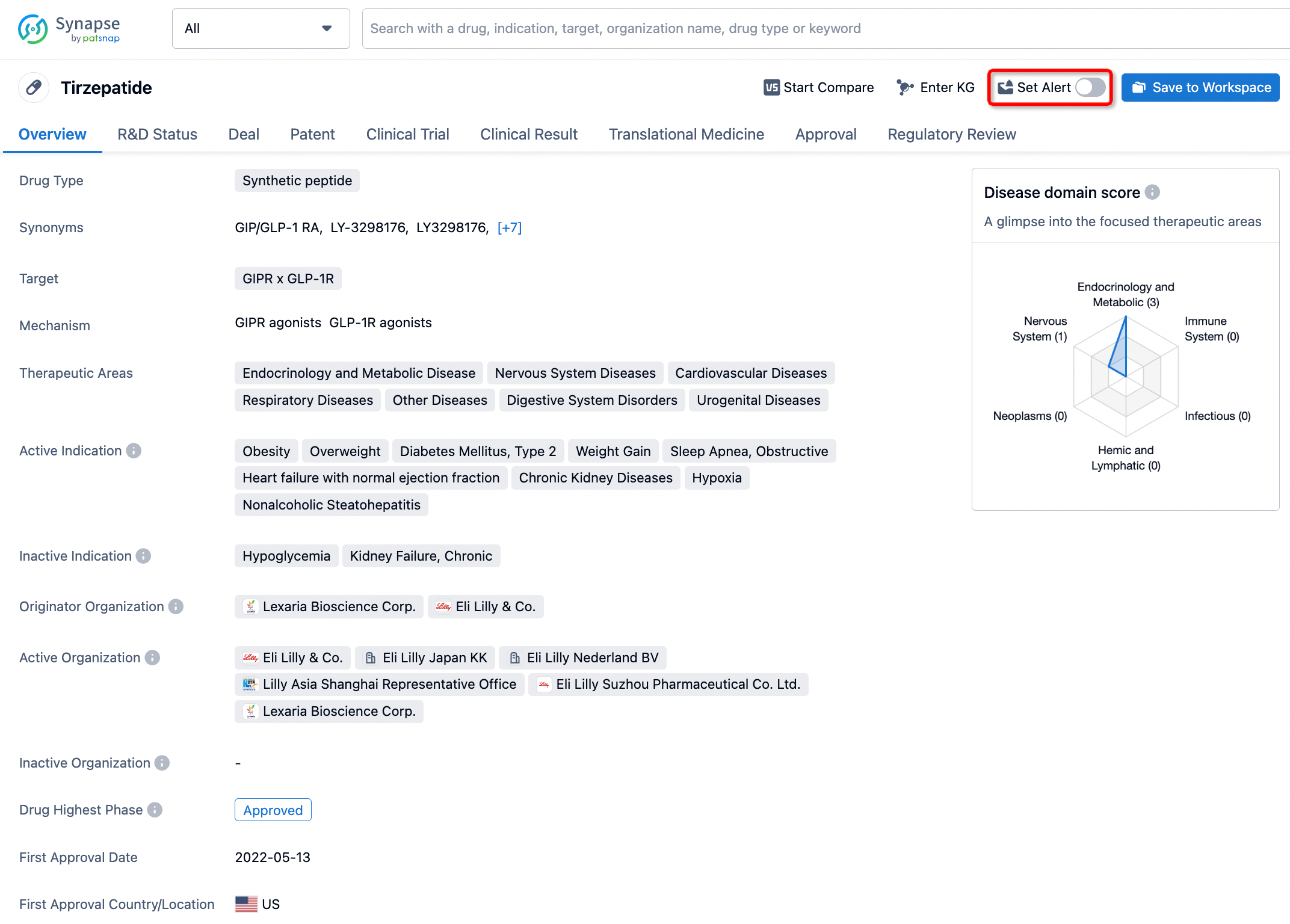
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!