Advances in Clinical Research on D2 Receptor Antagonist
The D2 receptor is a type of dopamine receptor found in the human body. It plays a crucial role in various physiological and neurological processes.The D2 receptor is a postsynaptic receptor that is highly expressed in the striatum. The activation of D2R can result in the signal pathway related to cell differentiation, growth, metabolism, and apoptosis. D2R also plays a crucial role in dopamine neurotransmission and motor control.
As a G-protein coupled receptor, the D2 receptor is involved in the regulation of dopamine neurotransmission, which affects mood, motivation, and reward. Dysfunction of the D2 receptor has been implicated in several psychiatric disorders, including schizophrenia and addiction. Additionally, the D2 receptor is a target for antipsychotic medications, which work by blocking its activity to alleviate symptoms associated with psychosis. Understanding the role of the D2 receptor is essential for developing therapeutic interventions for psychiatric and neurological conditions.
D2 receptor Competitive Landscape
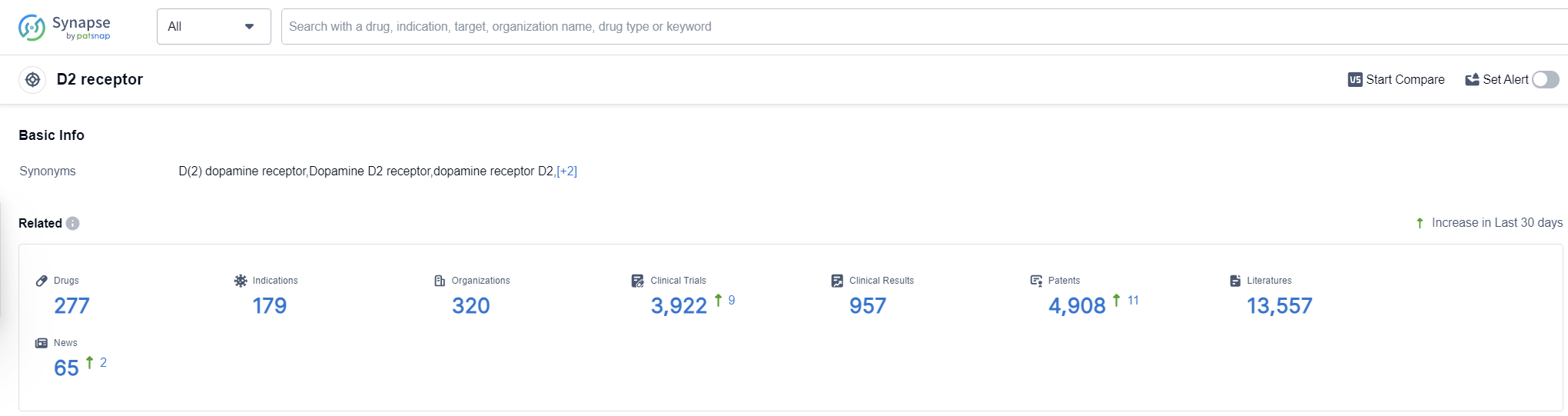
According to Patsnap Synapse, as of 14 Oct 2023, there are a total of 277 D2 receptor drugs worldwide, from 320 organizations, covering 179 indications, and conducting 3922 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target D2 receptor reveals a competitive landscape with multiple companies making progress in the development of drugs. Johnson & Johnson, Mitsubishi Chemical Group Corp., and Novartis AG have shown the highest stage of development, with multiple approved drugs. The most common indications for approved drugs are schizophrenia, bipolar disorder, bipolar I disorder, and Parkinson's disease.
Small molecule drugs dominate the development pipeline, indicating intense competition around innovative drugs. Synthetic peptides and fusion proteins also show some progress. The United States, Japan, China, and the European Union are the leading countries/locations in the development of drugs targeting the D2 receptor, with China showing significant progress.
Overall, the target D2 receptor presents opportunities for further research and development, particularly in indications such as schizophrenia, bipolar disorder, and Parkinson's disease. The competitive landscape is dynamic, with companies and countries actively contributing to the advancement of drugs targeting the D2 receptor.
Approved for market D2 receptor antagonist: Domperidone Maleate
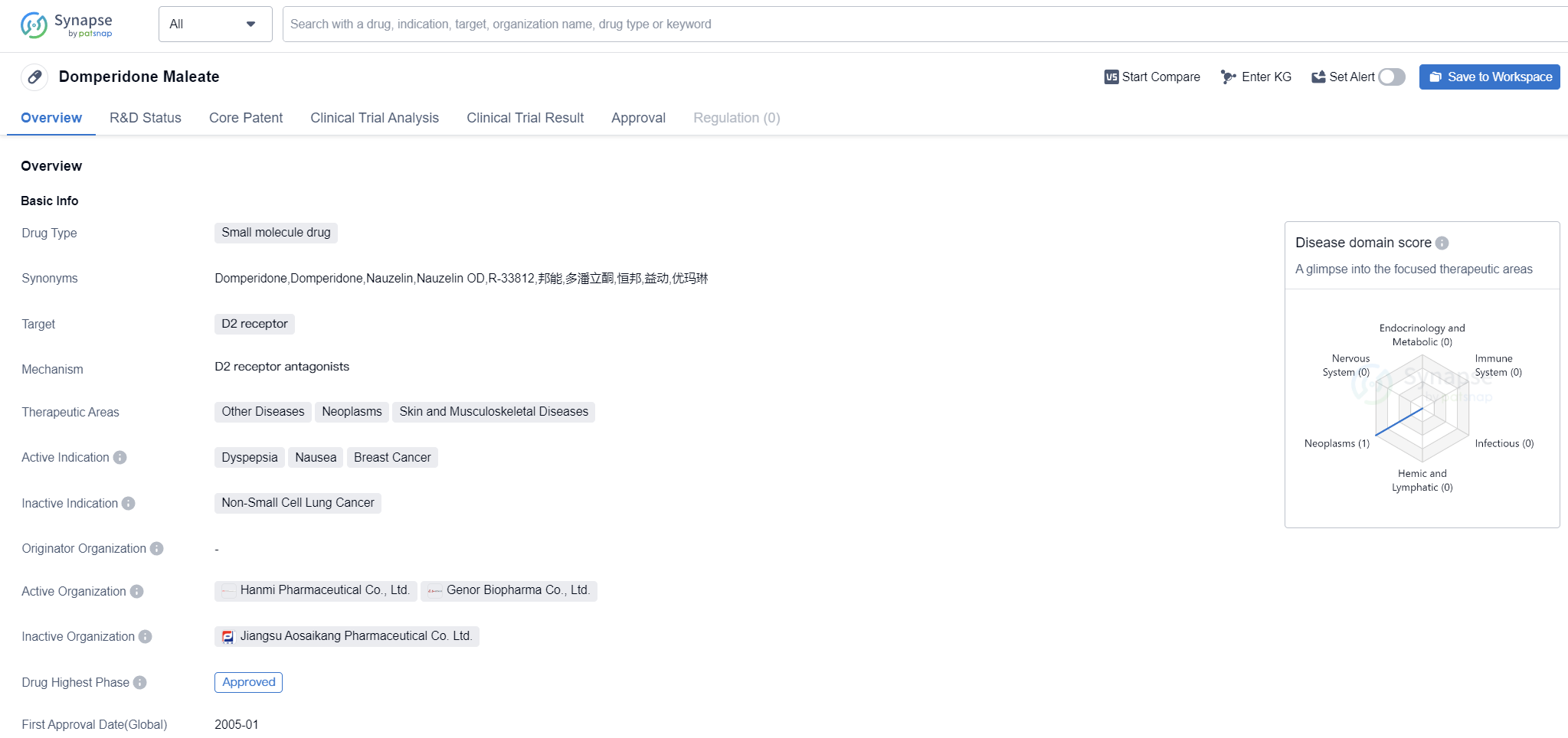
Domperidone Maleate is a small molecule drug that targets the D2 receptor. It is primarily used in the treatment of dyspepsia, nausea, and breast cancer. Additionally, it has therapeutic potential in other diseases, neoplasms, and skin and musculoskeletal diseases.
The drug has received approval for use in both global and Chinese markets. Its highest phase of development, which is the phase at which it received approval, is approved in both regions. The global approval for Domperidone Maleate was granted in January 2005, with China being the first country/location to approve its use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Domperidone Maleate is commonly prescribed for dyspepsia, a condition characterized by indigestion, discomfort, and pain in the upper abdomen. It helps alleviate symptoms such as bloating, belching, and heartburn. Nausea, another indication for this drug, refers to the feeling of sickness or the urge to vomit. Domperidone Maleate can effectively reduce nausea and provide relief to patients experiencing this symptom.
Breast cancer, a prevalent form of cancer among women, is also an active indication for Domperidone Maleate. It is used as part of the treatment regimen for breast cancer patients, although its specific role and efficacy in this context may require further investigation.
In addition to its approved indications, Domperidone Maleate shows potential in treating other diseases, neoplasms, and skin and musculoskeletal diseases. However, further research and clinical trials are necessary to establish its effectiveness in these therapeutic areas.
Overall, Domperidone Maleate is a small molecule drug that targets the D2 receptor. It has been approved for use in the treatment of dyspepsia, nausea, and breast cancer. Its approval in both global and Chinese markets highlights its potential as a therapeutic option. However, more research is needed to explore its efficacy in other diseases and conditions.
Orally active potent D2 receptor antagonist: Mosapramine Hydrochloride
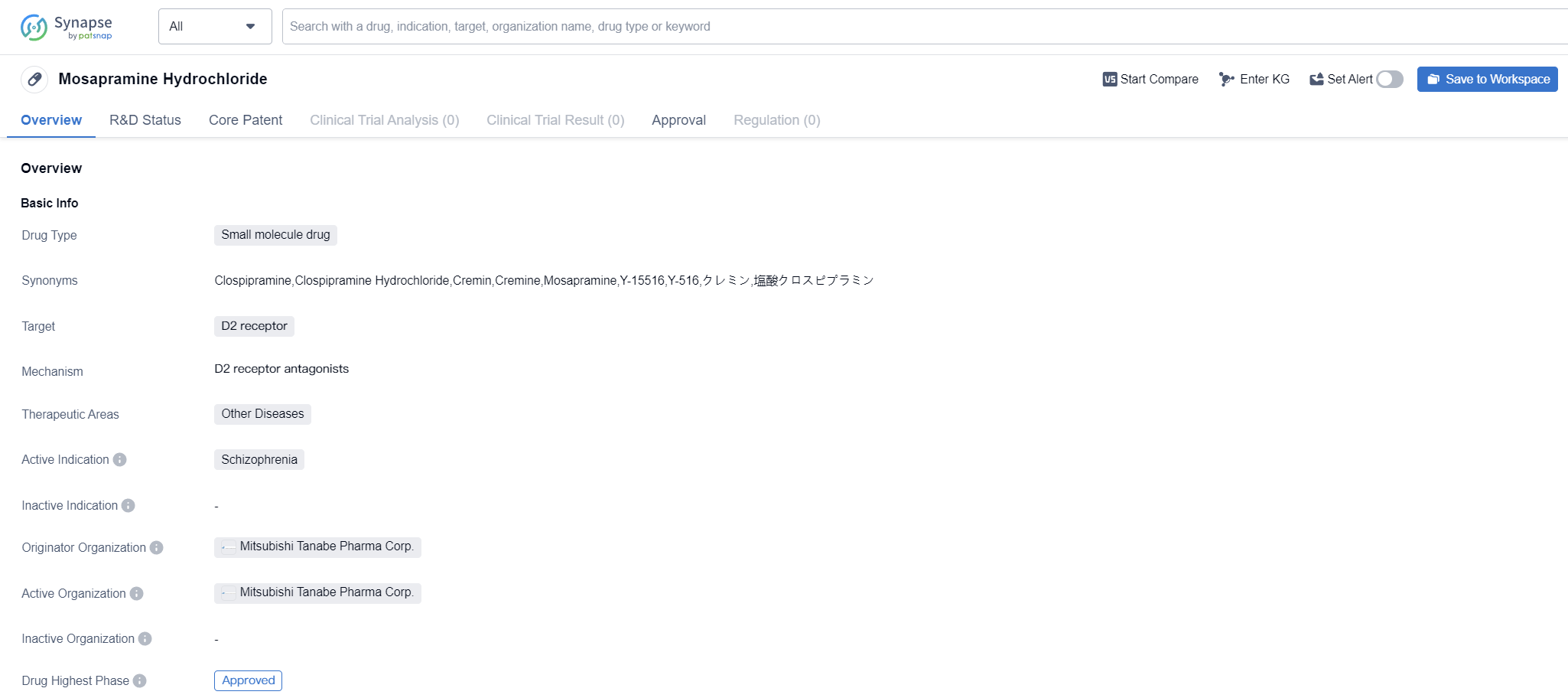
Mosapramine Hydrochloride is a small molecule drug that targets the D2 receptor. It is primarily used in the treatment of schizophrenia, a mental disorder characterized by abnormal social behavior and a failure to understand reality. The drug was first approved in Japan in January 1991 by Mitsubishi Tanabe Pharma Corp., making it the originator organization.
As a small molecule drug, Mosapramine Hydrochloride is designed to interact with specific receptors in the brain, particularly the D2 receptor. By targeting this receptor, the drug aims to modulate the levels of dopamine, a neurotransmitter involved in various brain functions. Schizophrenia is believed to be associated with an imbalance in dopamine levels, and Mosapramine Hydrochloride helps restore this balance, thereby alleviating the symptoms of the disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic area of Mosapramine Hydrochloride is categorized as "Other Diseases," indicating that it may have potential applications beyond schizophrenia. However, the information provided does not specify any additional indications or ongoing clinical trials for this drug.
Since its first approval in Japan in 1991, Mosapramine Hydrochloride has not progressed beyond the approved phase globally. This suggests that it may not have received regulatory approval in other countries or failed to demonstrate significant efficacy or safety in further clinical trials.
In summary, Mosapramine Hydrochloride is a small molecule drug developed by Mitsubishi Tanabe Pharma Corp. It targets the D2 receptor and is primarily used for the treatment of schizophrenia. While it was approved in Japan in 1991, it has not progressed beyond the approved phase globally. The drug's therapeutic area is categorized as "Other Diseases," indicating potential applications beyond schizophrenia, although no specific indications or ongoing trials are mentioned.