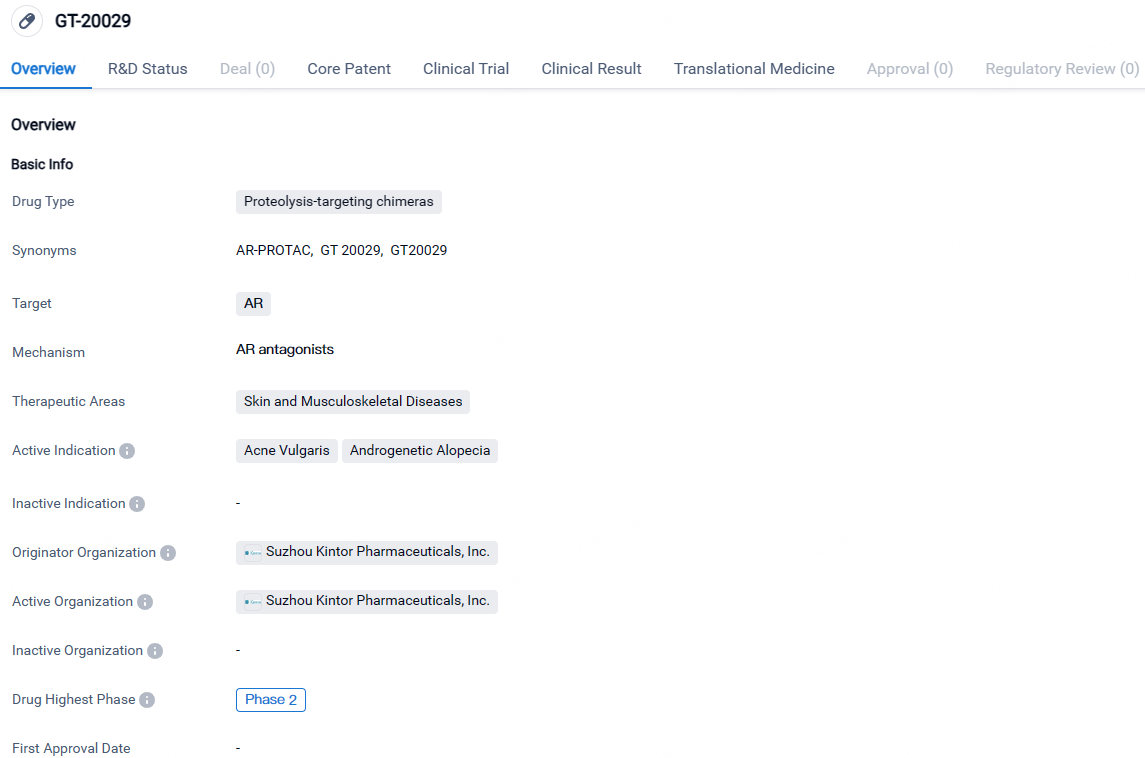
AGA China Phase II Study GT20029 Successfully Meets Its Primary Goal
Kintor Pharmaceutical Limited, a biotech firm in the clinical stage focused on creating novel small molecules and biologic treatments, disclosed that its domestically developed, pioneering proteolysis targeting chimera compound GT20029, which targets the androgen receptor (“AR”), has successfully met the primary endpoint in a phase II trial in China. This trial, aiming to treat male androgenetic alopecia with GT20029, showed statistically significant outcomes that were both clinically significant and demonstrated a favorable safety and tolerability profile.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Following the outcome of the Phase II Clinical Trial, the organization is gearing up to advance clinical ventures for GT20029, including launching a Phase III clinical trial within China and a Phase II study in the U.S. specifically for male AGA. Furthermore, preparations are underway for a Phase II study on GT20029 targeting the treatment of acne.
The Phase II Clinical Trial, a multicenter, randomized, double-blind, placebo-controlled analysis, seeks to assess the effectiveness and safety of GT20029 in treating male AGA and to establish the optimal dosage for the subsequent Phase III trial. This study spans across 12 clinical research facilities in China, with Professor Yang Qinping from Fudan University Huashan Hospital serving as the chief investigator.
The key measure for success in this study is the mean difference in non-vellus hair count at the target area at the 12-week mark compared to the placebo group. Safety measures considered include adverse reactions, laboratory diagnostics, subjective analyses of the topical drug, and dermatological evaluations. The study includes 180 male AGA subjects divided into groups receiving once daily and twice weekly doses. Each cohort has control and test groups receiving doses of either 0.5% or 1%. The finding reveals:
In terms of safety, GT20029 applied topically showed high tolerability and safety, with adverse event rates during the study similar to those of the placebo group. Additionally, no adverse sexual effects were reported.
GT20029 is a pioneering dermatology-focused topical novel AR degrader developed through the proprietary PROTAC platform of the company. It marks the first topical PROTAC entity to have concluded Phase I clinical trials in both China and the U.S. GT20029 targets AR proteins for degradation by facilitating their recruitment to E3 ubiquitin ligase. It primarily impacts peripheral skin tissues, thus minimizing systemic distribution and decreasing the AR sensitivity to androgens in the local sebaceous glands of hair follicles. Therefore, it is being developed by the Group to treat AGA as well as acne.
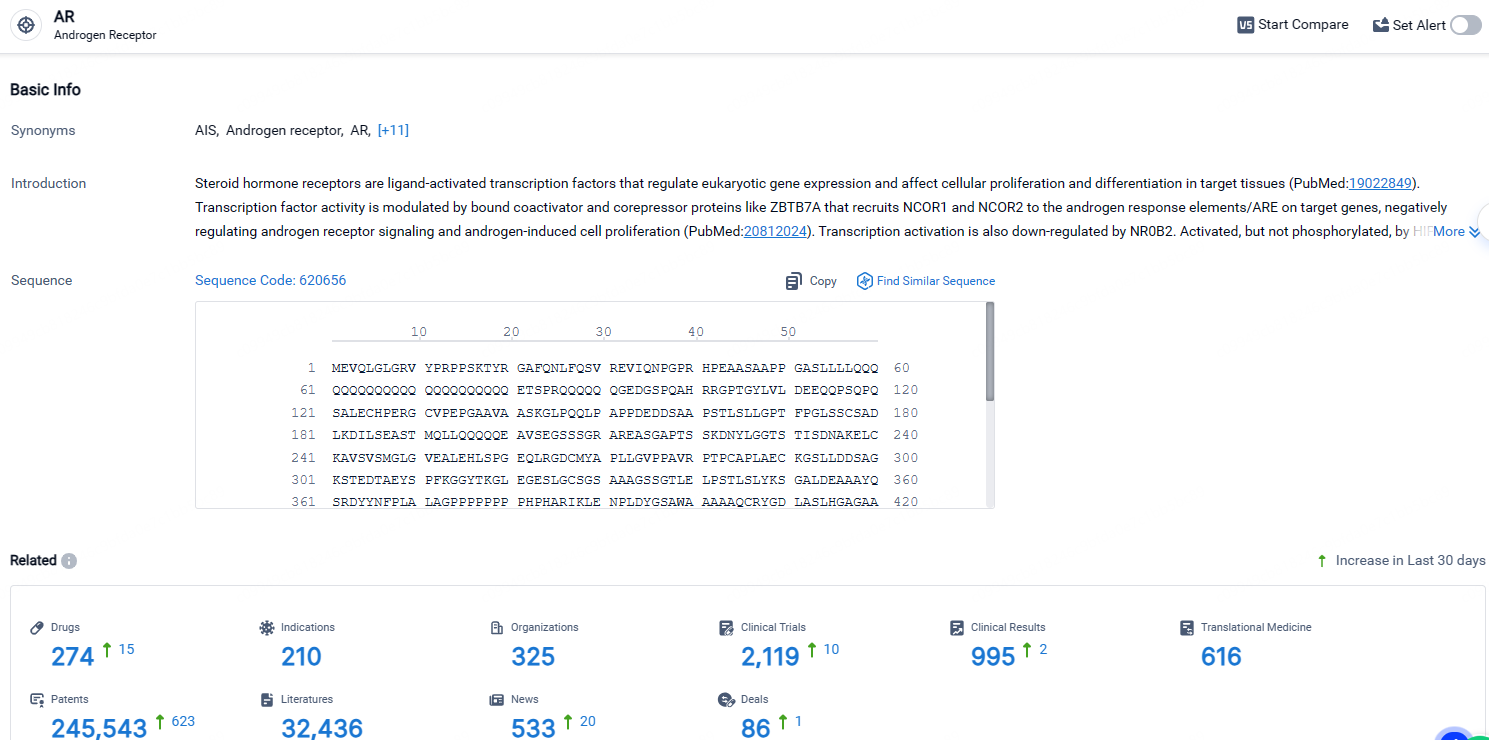
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 23, 2024, there are 274 investigational drugs for the AR receptor, including 210 indications, 325 R&D institutions involved, with related clinical trials reaching 2119, and as many as 245543 patents.
GT-20029 targets the androgen receptor and is intended for the treatment of skin and musculoskeletal diseases, specifically acne vulgaris and androgenetic alopecia. The drug is currently in Phase 2 of clinical development, both globally and in China, indicating promising results in earlier stages of testing.