Agomab Begins Phase I Trial of AGMB-447 in Healthy Subjects and IPF Patients
Agomab Therapeutics NV has revealed the initiation of a pioneering Phase 1 clinical trial by administering the initial dose of AGMB-447 to a participant. This novel small molecule, with a restrictive function to the lungs when inhaled, acts as an inhibitor to ALK5. The ongoing trial is assessing the effects of AGMB-447 both in individuals without underlying health conditions and in individuals diagnosed with idiopathic pulmonary fibrosis.
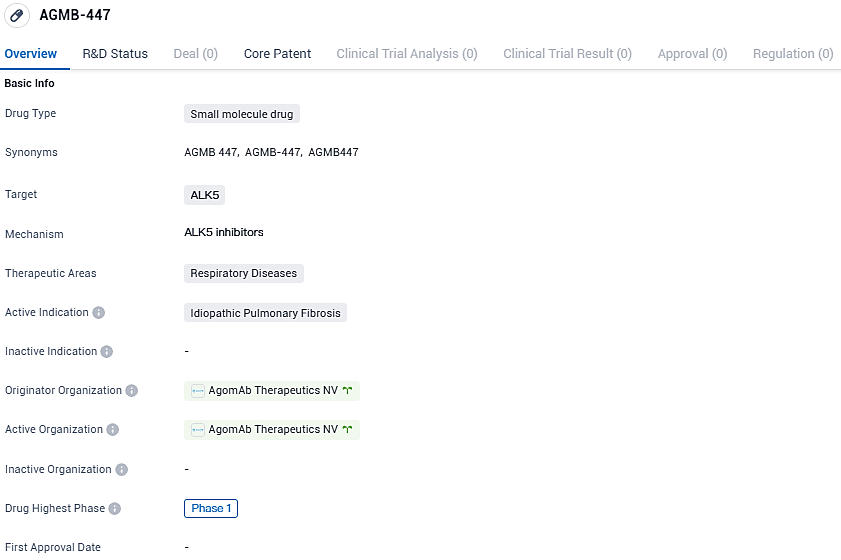
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial clinical study for AGMB-447 encompasses an evaluation of both a single dose escalation and multiple dose escalations in healthy volunteers, with subsequent administrations of AGMB-447 to individuals with IPF over a duration of 14 days. This clinical study is being carried out with a combined total of 76 participants and IPF sufferers in the United Kingdom. The main objectives of the study are to assess the drug's safety and patient comfort. Additional study aims involve the examination of AGMB-447's absorption and distribution within the body, as well as at the site of action.
"With the initiation of clinical testing for AGMB-447, Agomab's second compound targeting ALK5, we are embarking on a thrilling journey and reaching a significant stage," commented Andrea Sáez, Chief Development Officer at Agomab Therapeutics. "This drug candidate holds the promise of being a vital advancement in the treatment arsenal for individuals enduring IPF and additional lung-related fibrotic conditions."
Andrea Sáez also noted, "Owing to its lung-centric distribution, AGMB-447 is designed to specifically and yet gently intervene with TGFβ, a crucial contributor to fibrosis, within the lungs where it's needed most."
At present, AGMB-447 remains an experimental therapy and has yet to garner approval from health regulatory agencies. Its effectiveness and safety record are still under review.
Defined as a compound specifically targeting the lungs for the purpose of inhibiting ALK5, AGMB-447 is intended for the management of Idiopathic Pulmonary Fibrosis along with other fibrotic diseases affecting the respiratory system. AGMB-447 has been meticulously crafted to deliver a powerful yet careful inhibition of ALK5 within the pulmonary system, while its swift breakdown by hydrolysis in the bloodstream minimizes the likelihood of significant exposure throughout the body.
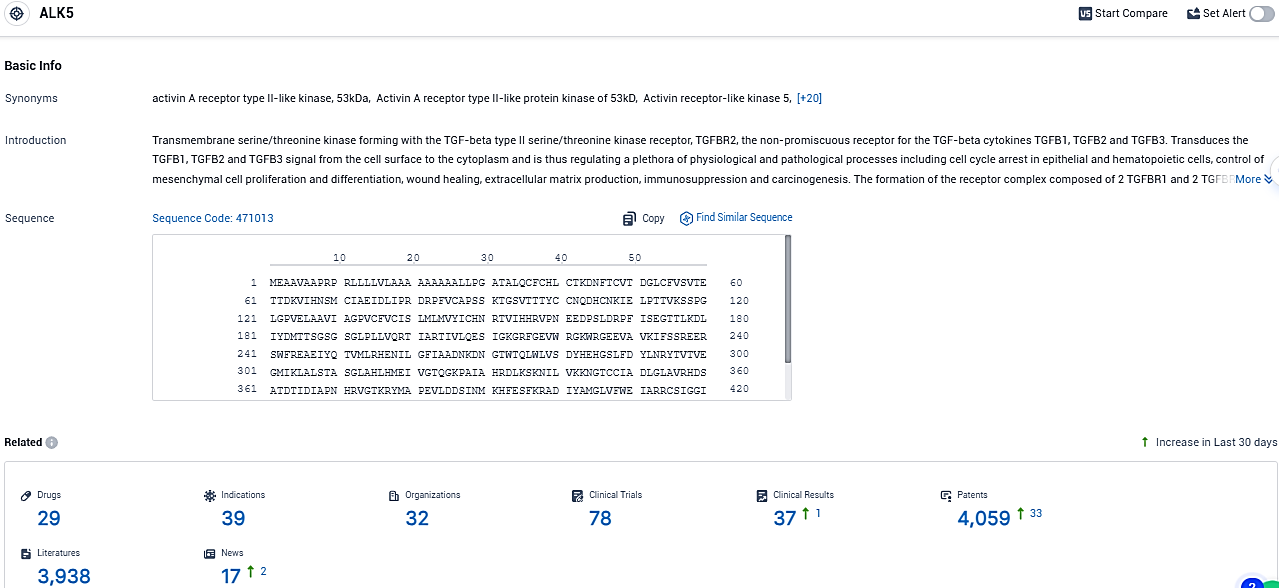
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 23, 2023, there are 29 investigational drugs for the ALK5 target, including 39 indications, 32 R&D institutions involved, with related clinical trials reaching 78, and as many as 4059 patents.
AGMB-447 is a small molecule drug being developed for the treatment of idiopathic pulmonary fibrosis. By targeting ALK5, the drug aims to modulate the TGF-β pathway and potentially inhibit the formation of scar tissue in the lungs. Currently in Phase 1 of clinical development, AGMB-447 holds promise as a potential therapeutic option for patients suffering from this debilitating respiratory disease.