Decoding Cadonilimab: a comprehensive study of its R&D trends and its clinical results in 2023 ESMO_ASIA
The recurrence rate of hepatocellular carcinoma (HCC) remains high and multinodular HCC is a well-defined high-risk factor. At the 2023 ESMO_ASIA , the latest clinical results of Cadonilimab were presented, demonstrating its potential therapeutic effect on HCC and laying a solid foundation for the next wave of research.
Cadonilimab's R&D Progress
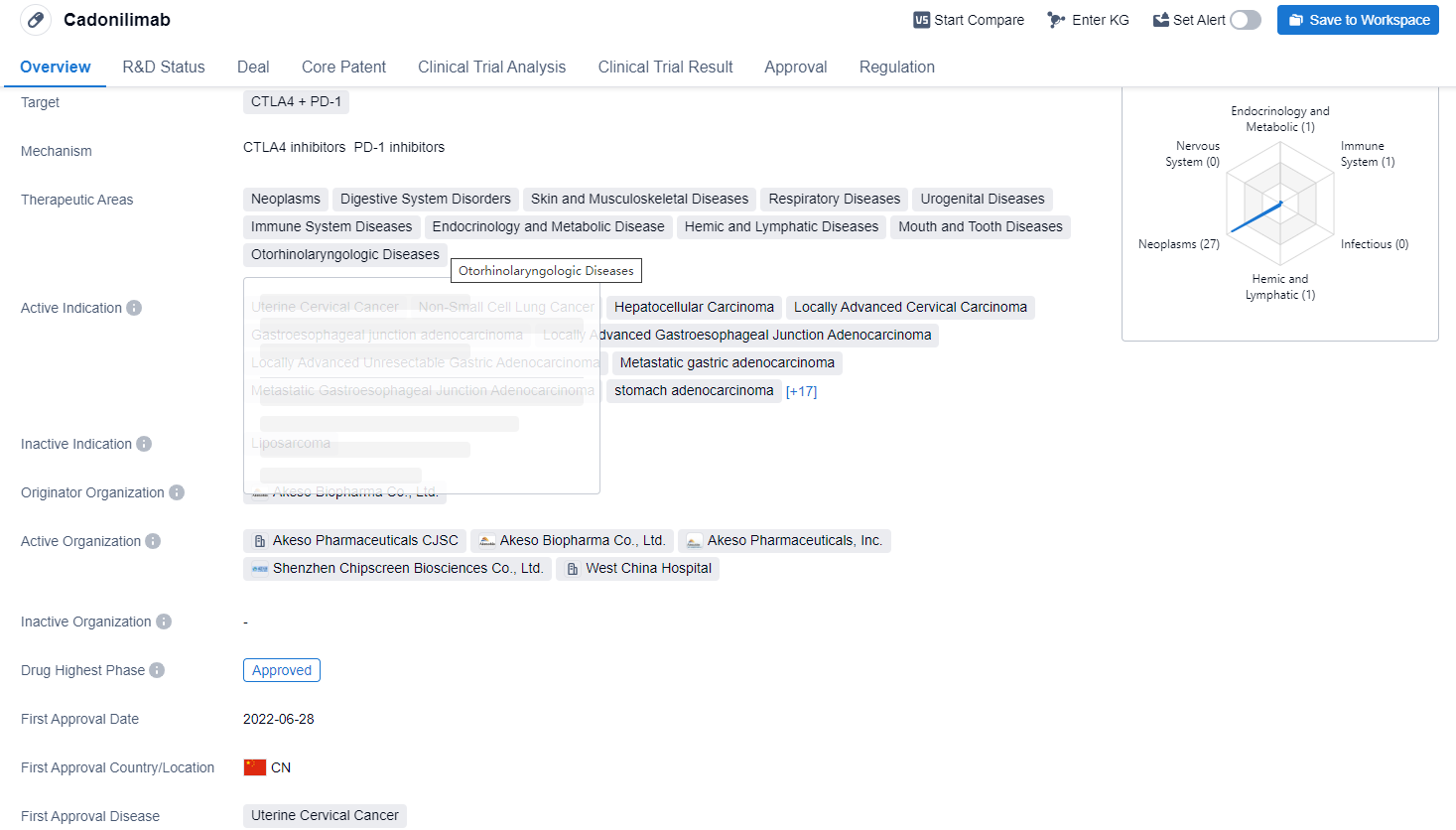
Cadonilimab is a bispecific antibody drug that targets CTLA4 and PD-1. The drug is primarily used for the treatment of various neoplasms, including uterine cervical cancer, non-small cell lung cancer, hepatocellular carcinoma, and locally advanced cervical carcinoma. It is also indicated for digestive system disorders, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, immune system diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, mouth and tooth diseases, and otorhinolaryngologic diseases.
According to the Patsnap Synapse, Cadonilimab has been developed by Akeso Biopharma Co., Ltd. and has received approval in China. And the clinical trial distributions for Cadonilimab are primarily in the United States, China and Australia. The key indication is Advanced Hepatocellular Carcinoma. 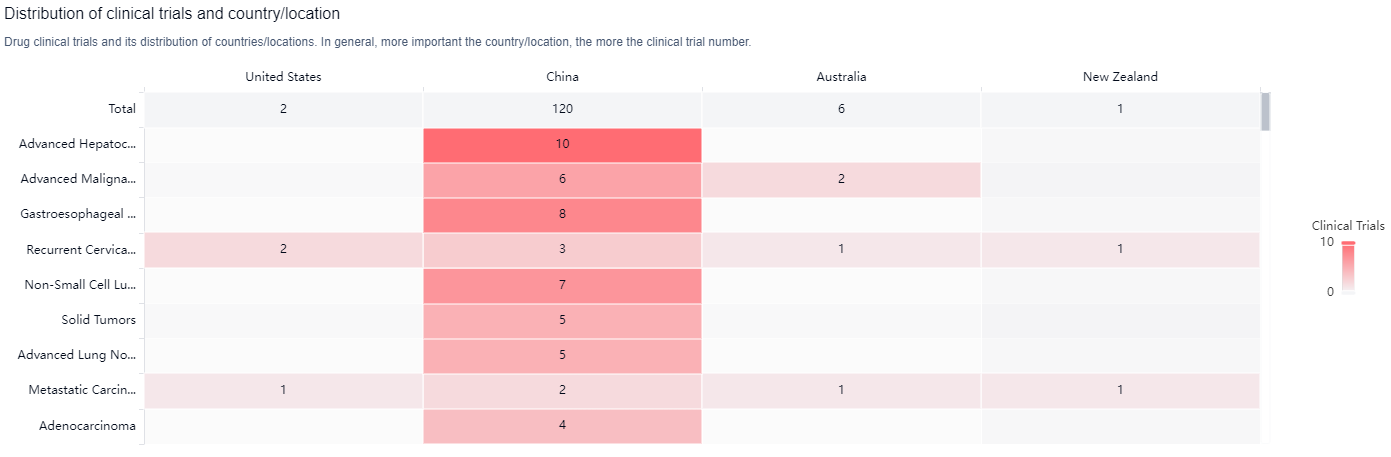
Detailed Clinical Result of Cadonilimab
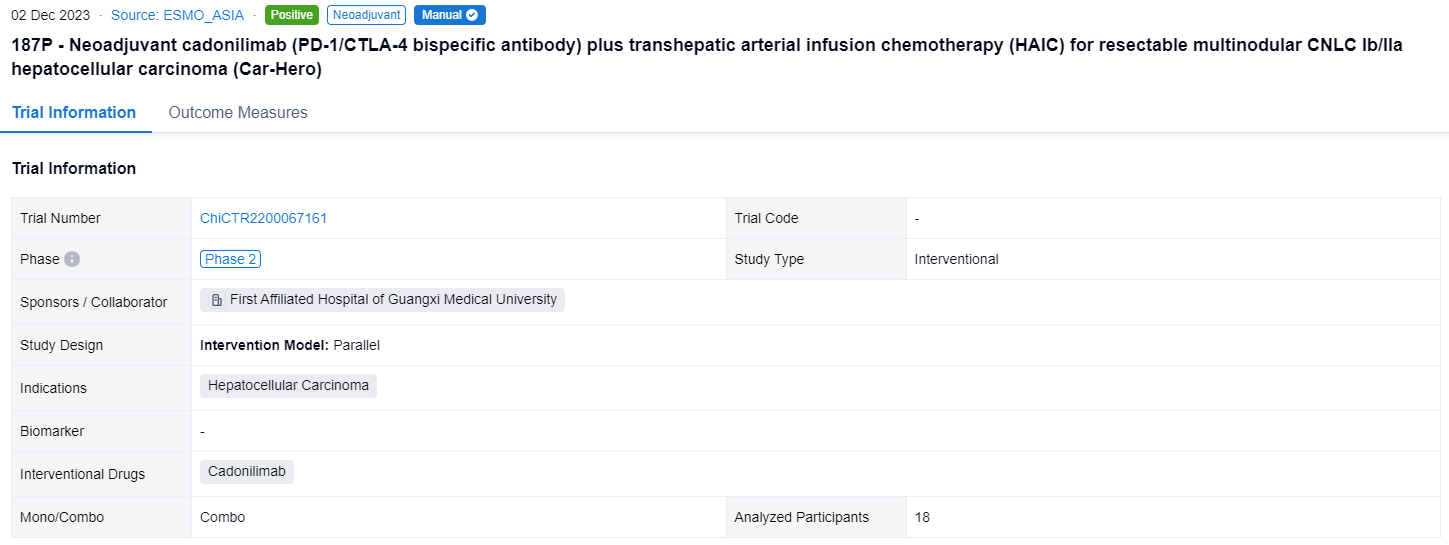
This this ongoing single-center, phase 2, open label, prospective cohort clinical trial (ChiCTR2200067161) was aimed to evaluate the safety and efficacy of cadonilimab plus HAIC as a neoadjuvant management for the resectable multinodular HCC with CNLC stage Ⅰb/Ⅱa.
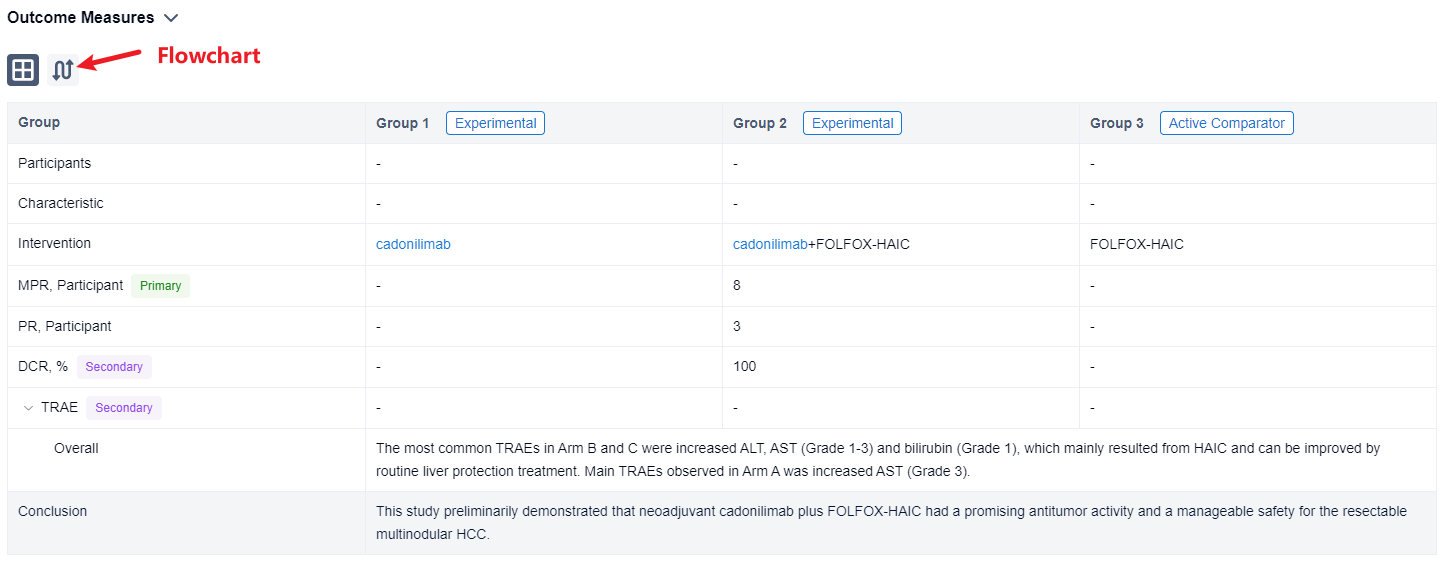
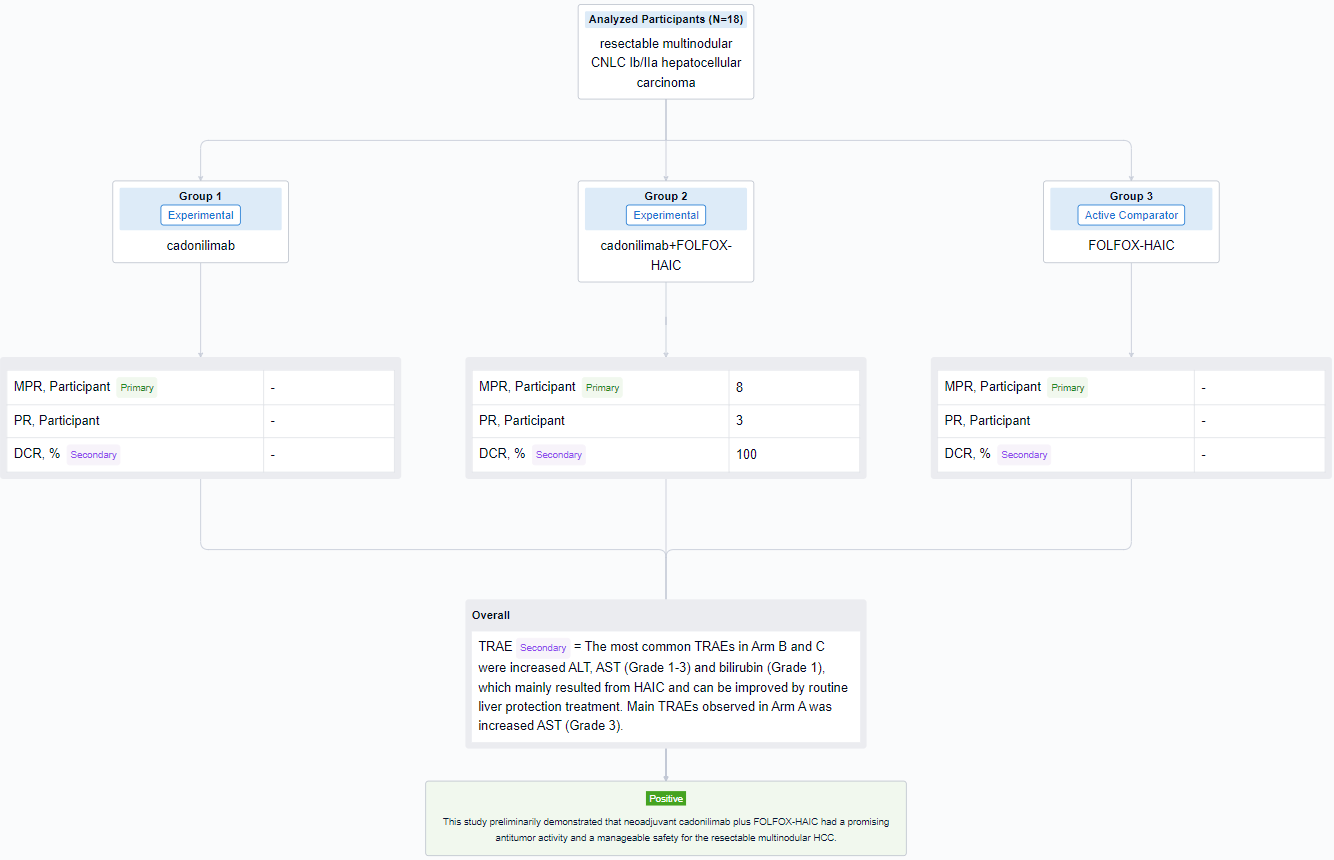
In this study, eligible patients (pts) were randomly assigned (1:1:1) to 3 arms and receive neoadjuvant therapy: (A) 2 cycles of cadonilimab (6mg/kg Q2W); (B) once HAIC with FOLFOX regimen followed by 2 cycles of cadonilimab; (C) once FOLFOX-HAIC. Pts receive scheduled surgery on day 21-28 and adjuvant HAIC one month after surgery. Primary endpoints were major pathologic response (MPR defined as ≤50% residual living tumor) and 1-year RFS rate. Secondary endpoints included ORR, DCR and TRAEs.
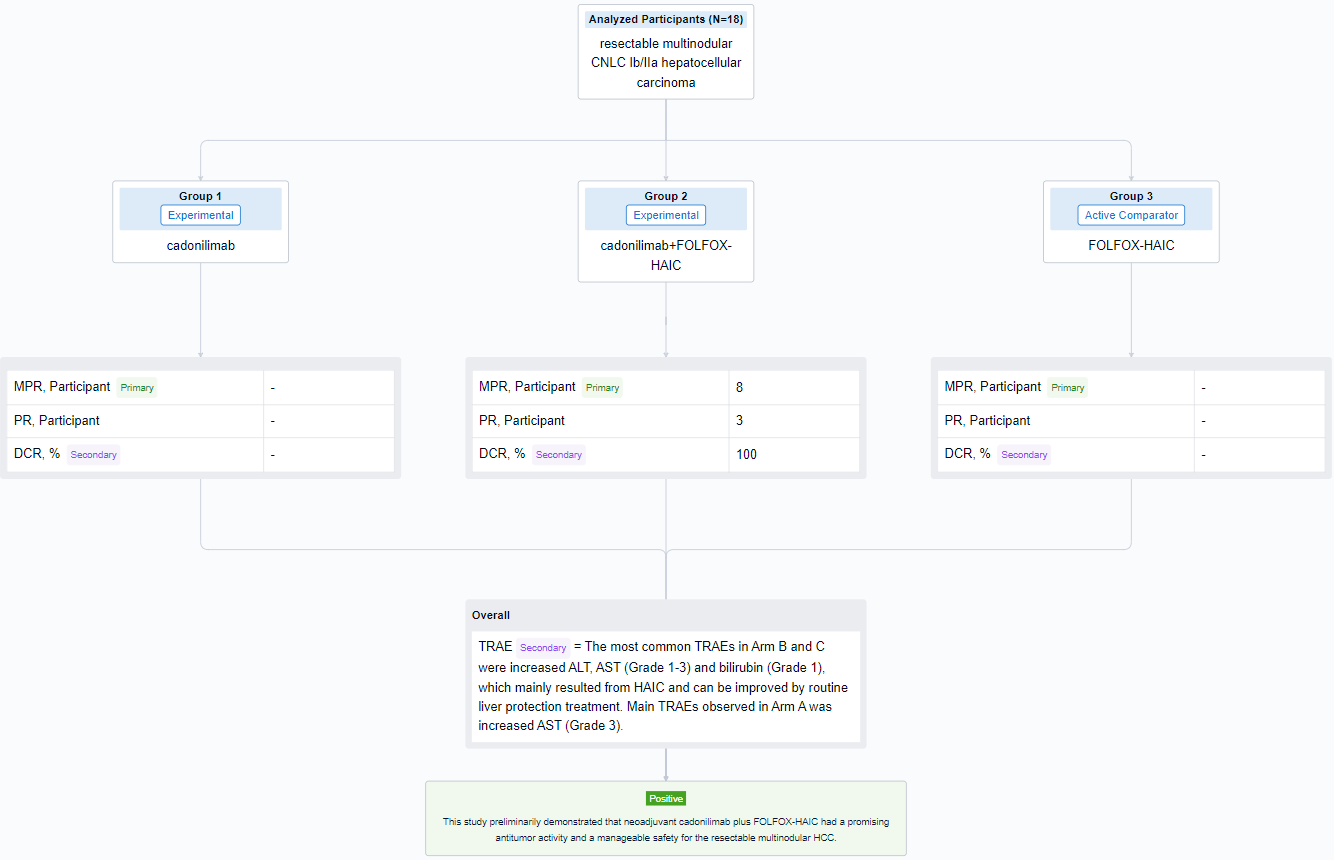
The result showed that from January 4 to August 20, 2023, 18 pts were enrolled and 16 pts have received scheming hepatectomy (5 pts in Arm A, 9 pts in Arm B, 2 pt in Arm C). Remarkably, almost all (8/9) pts in Arm B achieved MPR and some pts demonstrated focal heterogeneity—one lesion achieved pathologic complete response, while another got non-/partial response. No obvious tumor necrosis in Arm A and C, but the inflammatory infiltrating and fibrosis area seemed to increase in some HCC lesions. 3 pts in Arm B (3/9) achieved PR and DCR was 100% per RECIST 1.1. The most common TRAEs in Arm B and C were increased ALT, AST (Grade 1-3) and bilirubin (Grade 1), which mainly resulted from HAIC and can be improved by routine liver protection treatment. Main TRAEs observed in Arm A was increased AST (Grade 3). Two pts in Arm A and B delayed scheduled surgery for 3 weeks due to increased ALT/AST. One pt experienced postoperative controllable pneumonia which may relate to novel coronavirus (2019-nCoV) and cadonilimab. One pt experienced postoperative severe but curable bacterial hepatic abscess related to bilioenteric anastomosis and diabetes mellitus.
It can be concluded that neoadjuvant cadonilimab plus FOLFOX-HAIC had a promising antitumor activity and a manageable safety for the resectable multinodular HCC.
How to Easily View the Clinical Results Using Synapse Database?
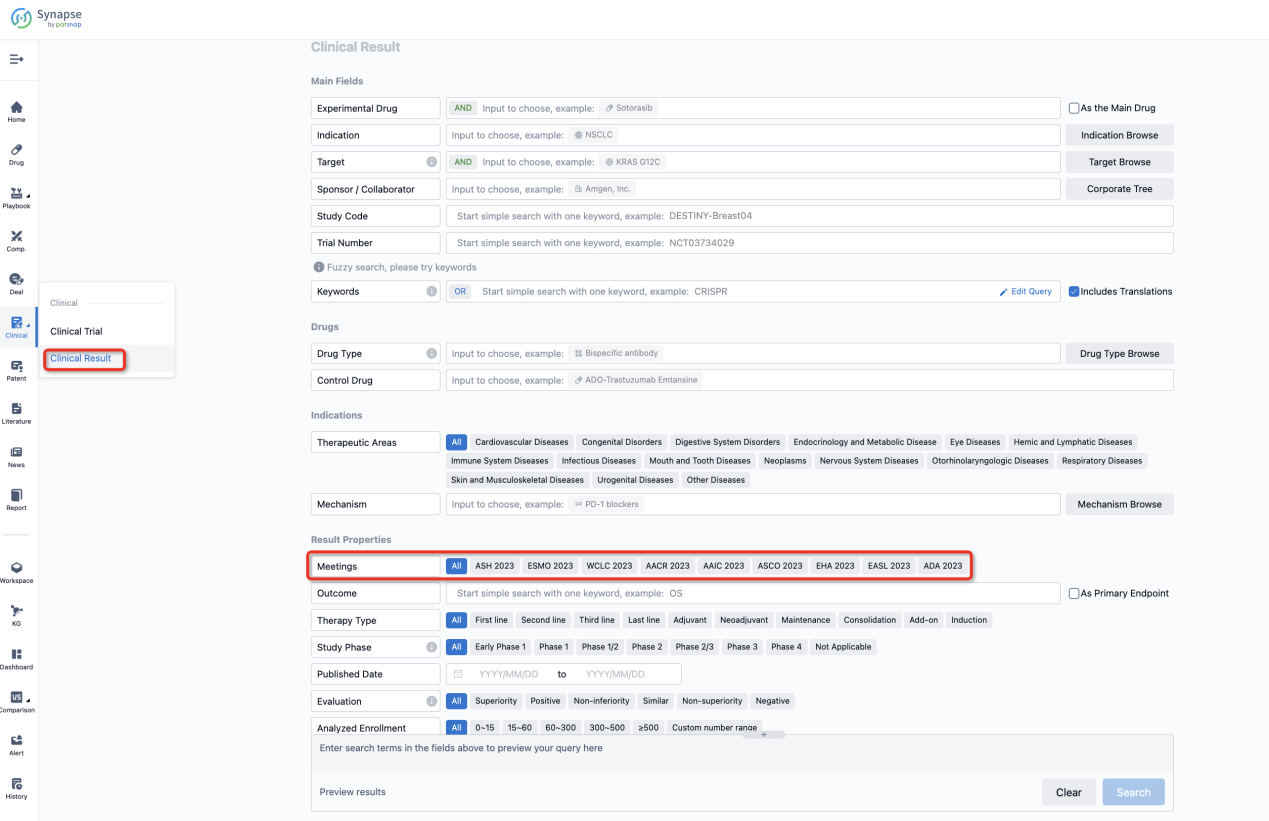
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
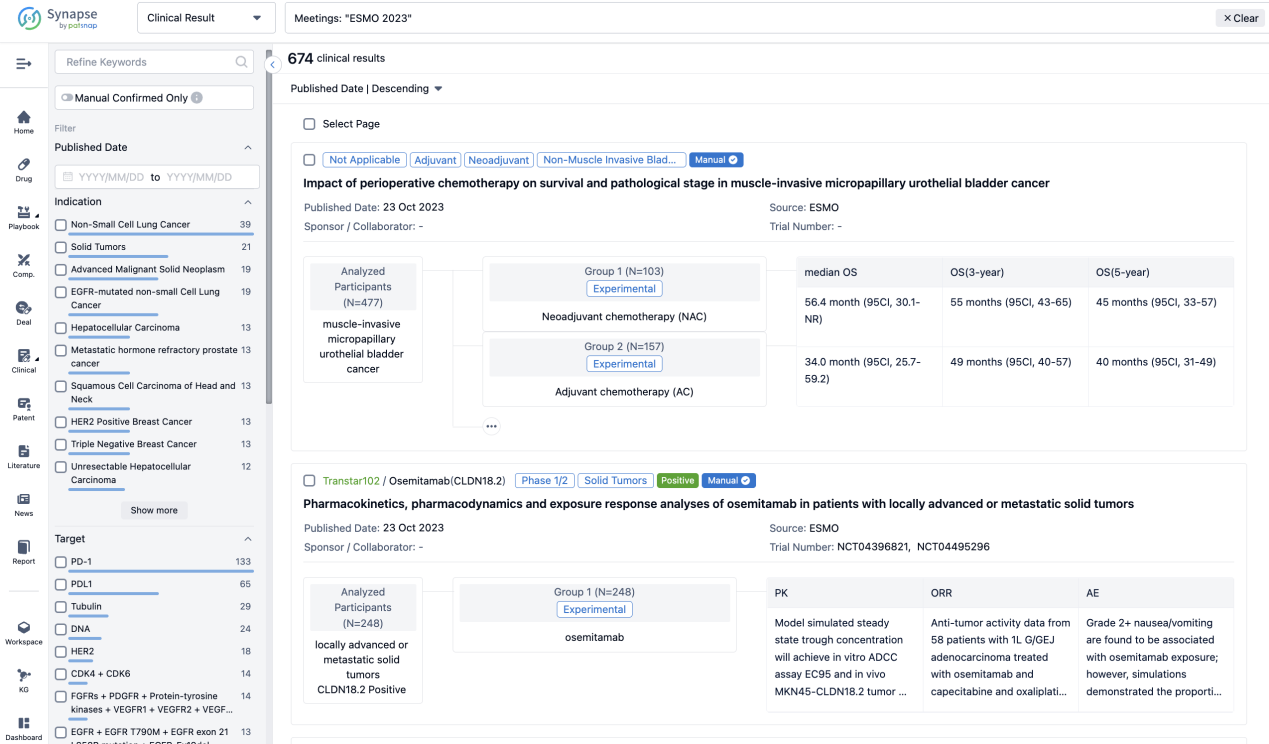
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
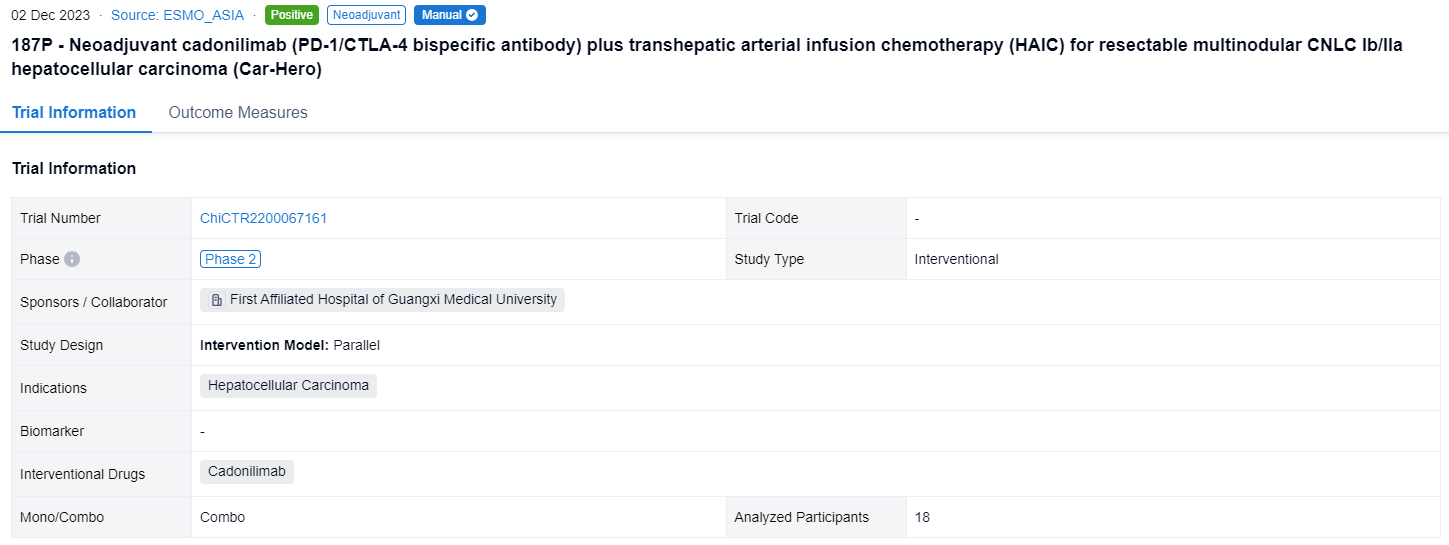
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!