Akeso Begins Phase 3 Trial of Ivonescimab with Ligufalimab vs. Pembrolizumab in First-Line HNSCC Treatment
Akeso Biopharma (9926.HK) is excited to report that the first patient has been enrolled in its randomized, controlled, multicenter Phase III trial (AK117-302) focusing on head and neck squamous cell carcinoma. This study is designed to assess the novel PD-1/VEGF bispecific antibody ivonescimab in conjunction with Akeso’s advanced CD47 monoclonal antibody ligufalimab (AK117) compared to pembrolizumab as a first-line treatment for PD-L1 positive (CPS≥1) recurrent/metastatic squamous cell carcinoma of the head and neck (R/M HNSCC).
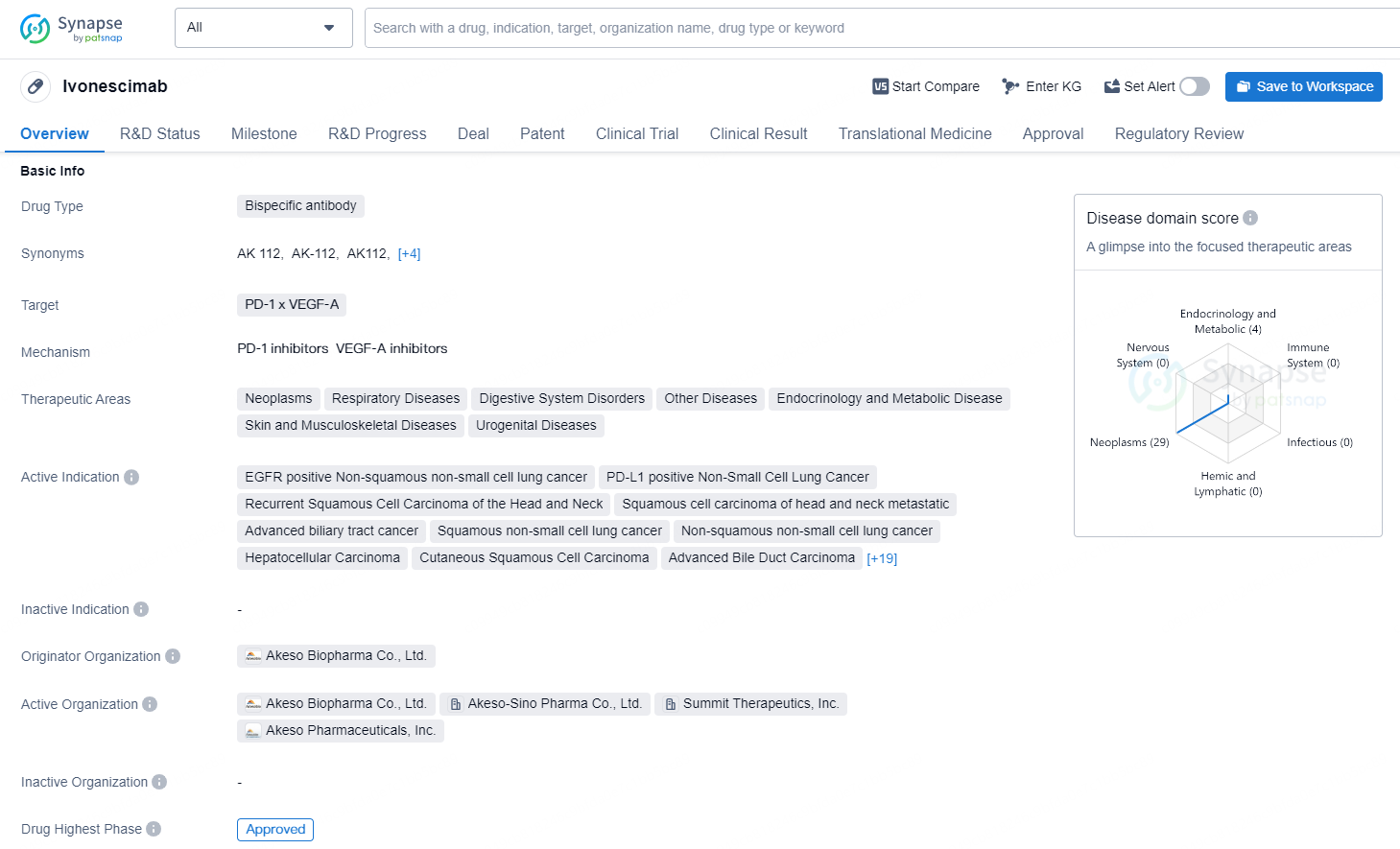
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The AK117-302 study marks a pivotal moment as the first Phase III clinical trial worldwide exploring a CD47 monoclonal antibody treatment specifically for solid tumors. This trial is the fifth Phase III assessment of ivonescimab, employing PD-1/L1 monoclonal antibody therapy as a reference control, and is the third Phase III comparison of ivonescimab against pembrolizumab. The AK117-302 study highlights Akeso’s dedication to pioneering advancements in cancer immunotherapy and setting a worldwide standard in cancer care. Additionally, it showcases our ability to enhance access to our product lineup for cancer patients across the globe through a well-planned clinical development strategy.
In 2022, the global incidence of head and neck cancer reached 770,000 new cases, with 84,000 of these occurring in China. Squamous cell carcinoma of the head and neck (HNSCC) makes up more than 90% of these cases. Regrettably, the five-year survival rate for individuals with recurrent/metastatic HNSCC (R/M HNSCC) is only 3.6%. Despite advancements in targeted therapies and immunotherapy, the median overall survival (OS) remains under one year.
Pembrolizumab has become the established first-line therapy for R/M HNSCC and is included in recommendations from both CSCO and NCCN guidelines. There is a significant unmet need for more effective treatments to enable HNSCC patients to attain extended survival.
At the upcoming European Society for Medical Oncology (ESMO) Congress in 2024, Akeso will present encouraging preliminary results from its combination therapy involving ivonescimab and ligufalimab. This combination has shown considerable tumor reduction and survival advantages, especially for HNSCC patients, who need swift tumor shrinkage. The initial efficacy results from this therapy have exceeded findings from earlier PD-1 studies, establishing it as a potentially new immunotherapeutic solution for HNSCC patients.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 31, 2024, there are 3 investigational drugs for the PD-1 x VEGF-A target, including 30 indications, 6 R&D institutions involved, with related clinical trials reaching 359, and as many as 1914 patents.
Ivonescimab is a bispecific antibody that targets PD-1 x VEGF-A and is used in various therapeutic areas including neoplasms, respiratory diseases, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. Its active indications include EGFR positive non-squamous non-small cell lung cancer, PD-L1 positive non-small cell lung cancer, recurrent squamous cell carcinoma of the head and neck, squamous cell carcinoma of head and neck metastatic, advanced biliary tract cancer, and several other advanced cancer types.