US FDA approves BLA for HLX14, a biosimilar to PROLIA/XGEVA (denosumab)
Shanghai Henlius Biotech, Inc. (2696.HK) and Organon (NYSE: OGN) revealed that the Biologic License Application (BLA) for HLX14, a biosimilar candidate for PROLIA/XGEVA (denosumab), has been accepted by the US Food and Drug Administration (FDA).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Denosumab has received approval across various countries and regions, marketed under multiple brand names for a range of indications, including the treatment of osteoporosis in postmenopausal women at elevated risk for fractures, among others.
In 2022, Henlius entered into a licensing and supply agreement with Organon, granting Organon exclusive rights to commercialize two biosimilar candidates, one of which is HLX14. The agreement spans markets including the United States, the European Union, and Canada, with the exception of China.
The filing is supported by data derived from several head-to-head studies of HLX14, encompassing comparative analytical quality assessments and two clinical trials. The first trial was a two-part phase 1 clinical study involving healthy adult males in China. The first part was an open-label, randomized, parallel-controlled pilot study designed to compare the pharmacokinetic (PK) parameters of HLX14 with EU-sourced PROLIA post-subcutaneous injection, providing foundational data for the design of the second part.
The second part was a double-blind, randomized, parallel-controlled, single-dose study consisting of four arms, aimed primarily at assessing the pharmacokinetic similarity of HLX14 against PROLIA sourced from the US, EU, and China following subcutaneous administration. The second trial was a randomized, double-blind, international multicenter phase 3 study that compared the efficacy, safety, tolerability, and immunogenicity of HLX14 with EU-sourced reference PROLIA in postmenopausal women with osteoporosis who are at a high risk of fractures.
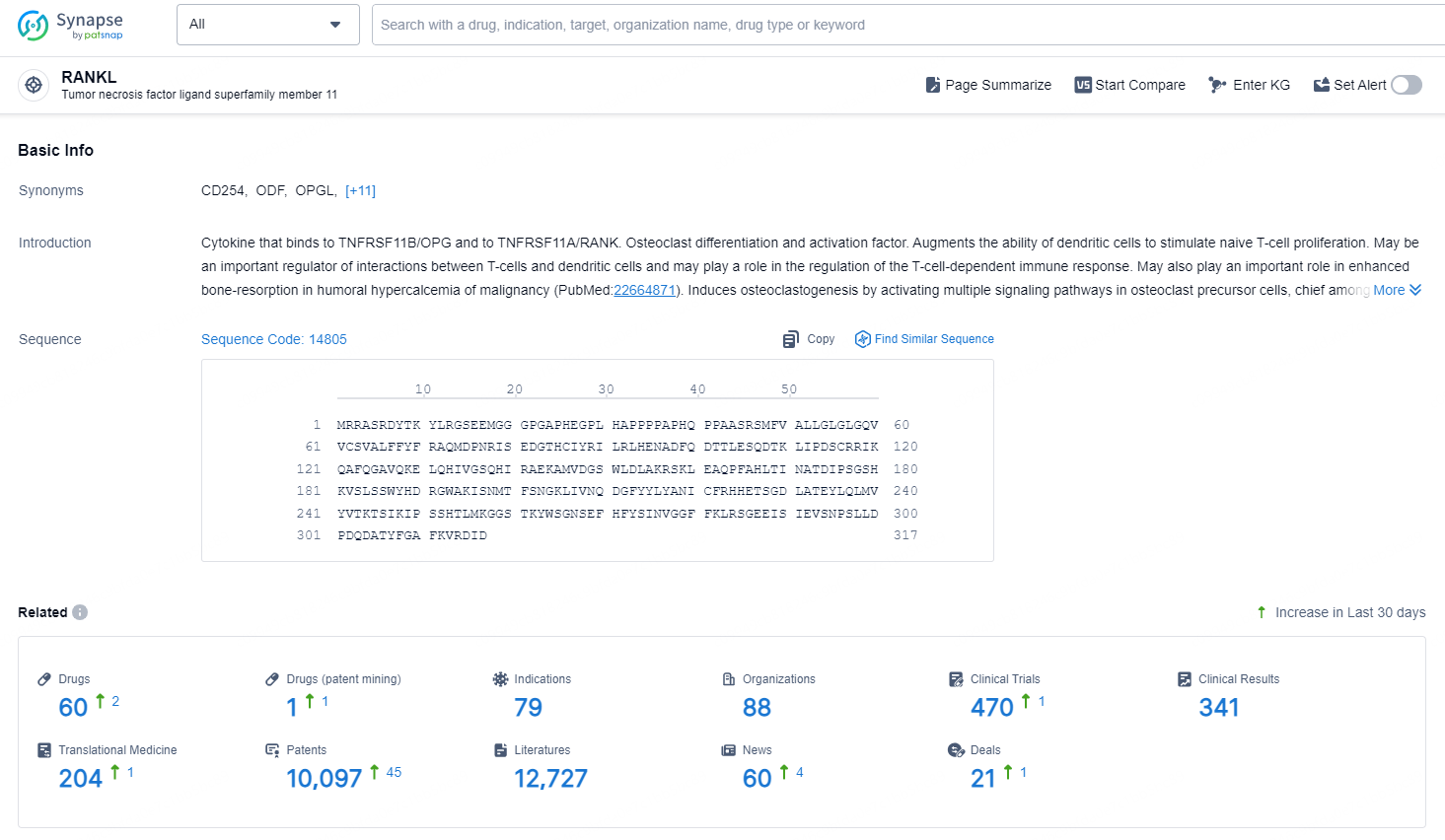
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 31, 2024, there are 60 investigational drug for the RANKL target, including 79 indications, 88 R&D institutions involved, with related clinical trials reaching 470, and as many as 10097 patents.
The Denosumab biosimilar (Shanghai Henlius) is a monoclonal antibody type of biosimilar drug that targets RANKL and is primarily used for the treatment of osteoporosis, postmenopausal fractures, and bone-related issues. It falls within the therapeutic areas of Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, and Other Diseases.