Exploring Amisulpride: Its Applications, Patents, and Impact on Psychiatric and Gastroenterological Disorders
Amisulpride is primarily used to treat psychiatric conditions such as schizophrenia. Additionally, it is also used in the management of nausea and vomiting, including postoperative symptoms. As a small molecule drug, it is designed to act specifically on the D2 and D3 receptors, which are known to play a role in various neurological and behavioral disorders.
The drug has achieved approval in both China and globally, indicating its widespread use and acceptance in the medical community. Its approval in 2004 suggests that it has been in use for over a decade, demonstrating a track record of safety and efficacy.Amisulpride's therapeutic areas include "Other Diseases," indicating its potential for treating a range of conditions beyond its primary indications. This versatility may contribute to its broader appeal and utilization in clinical practice.
Below, we will use the drug Amisulpride as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
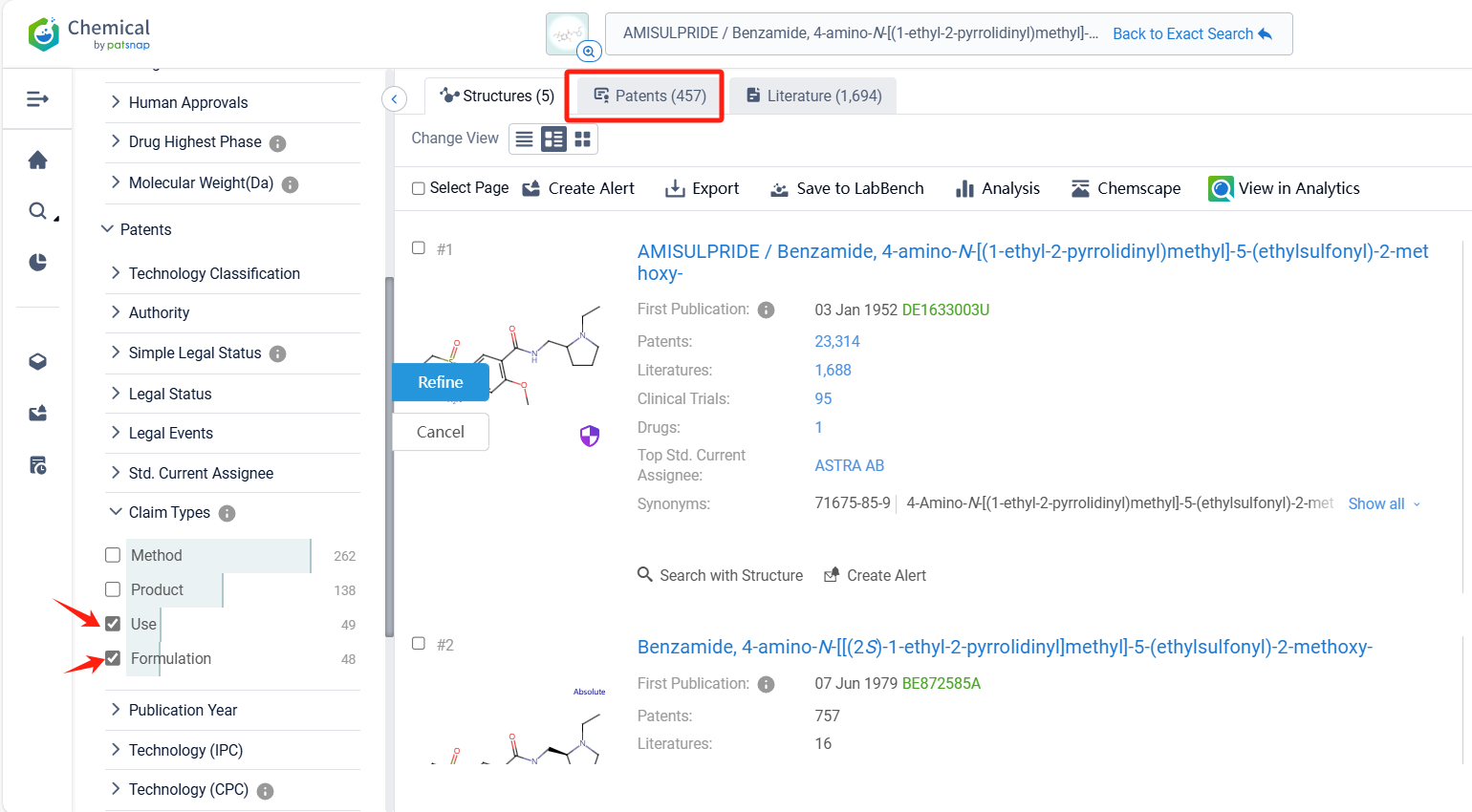
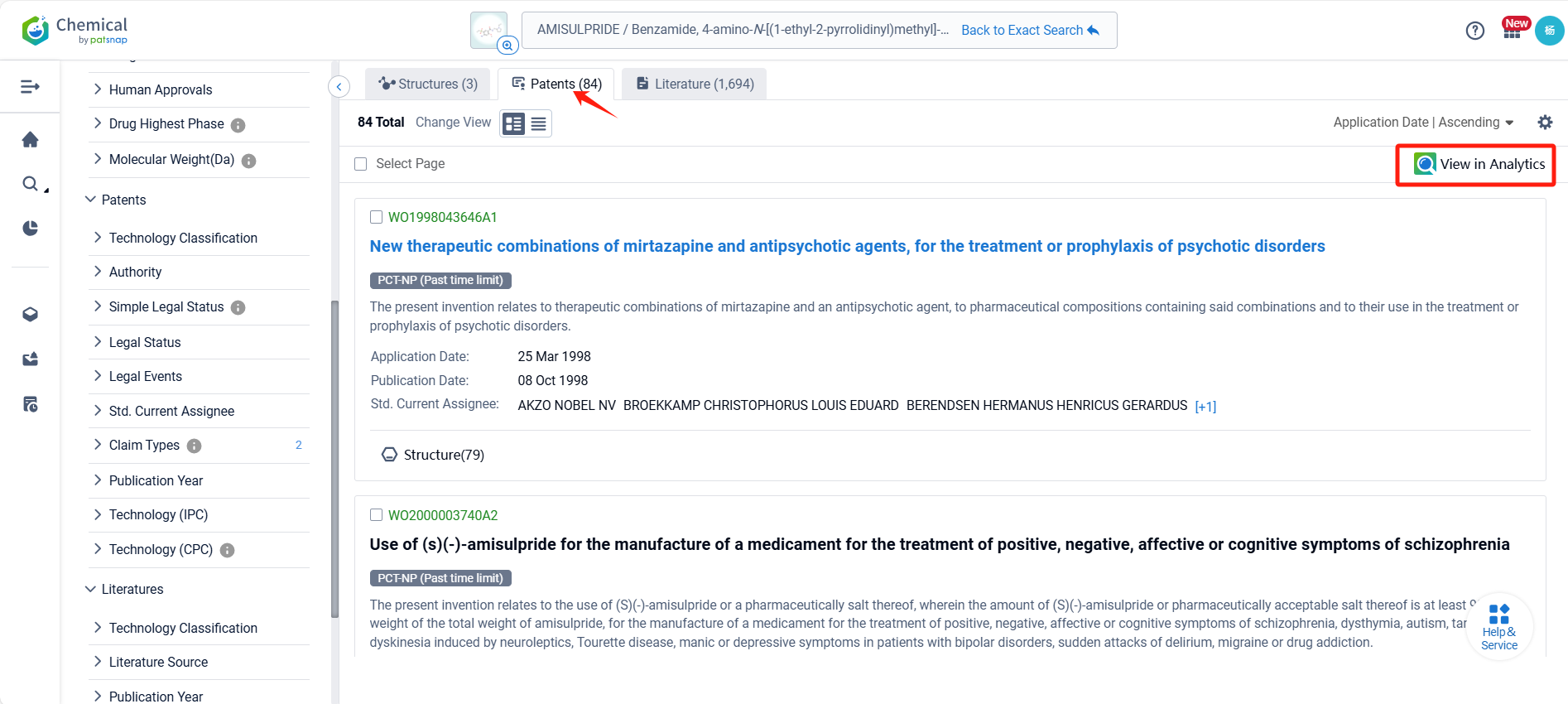
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Amisulpride(such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 457 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
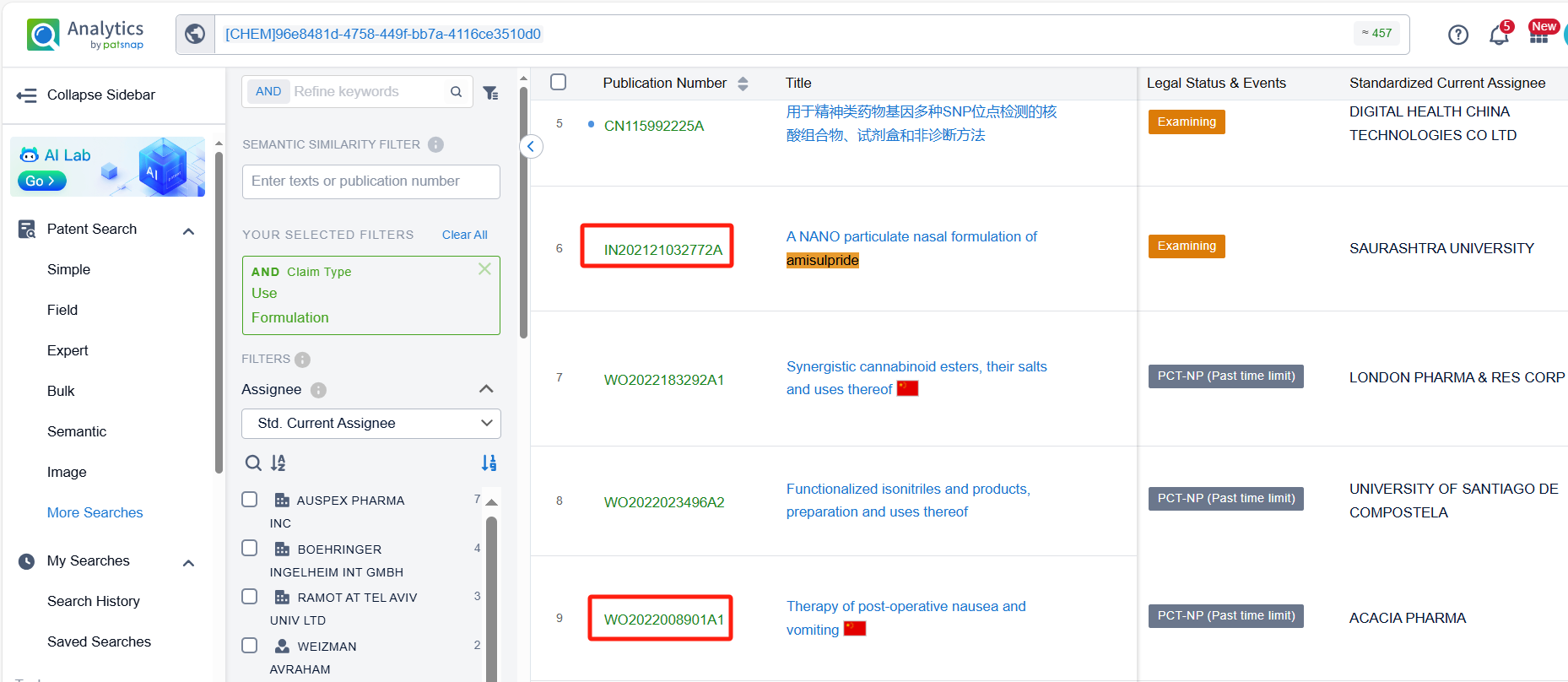
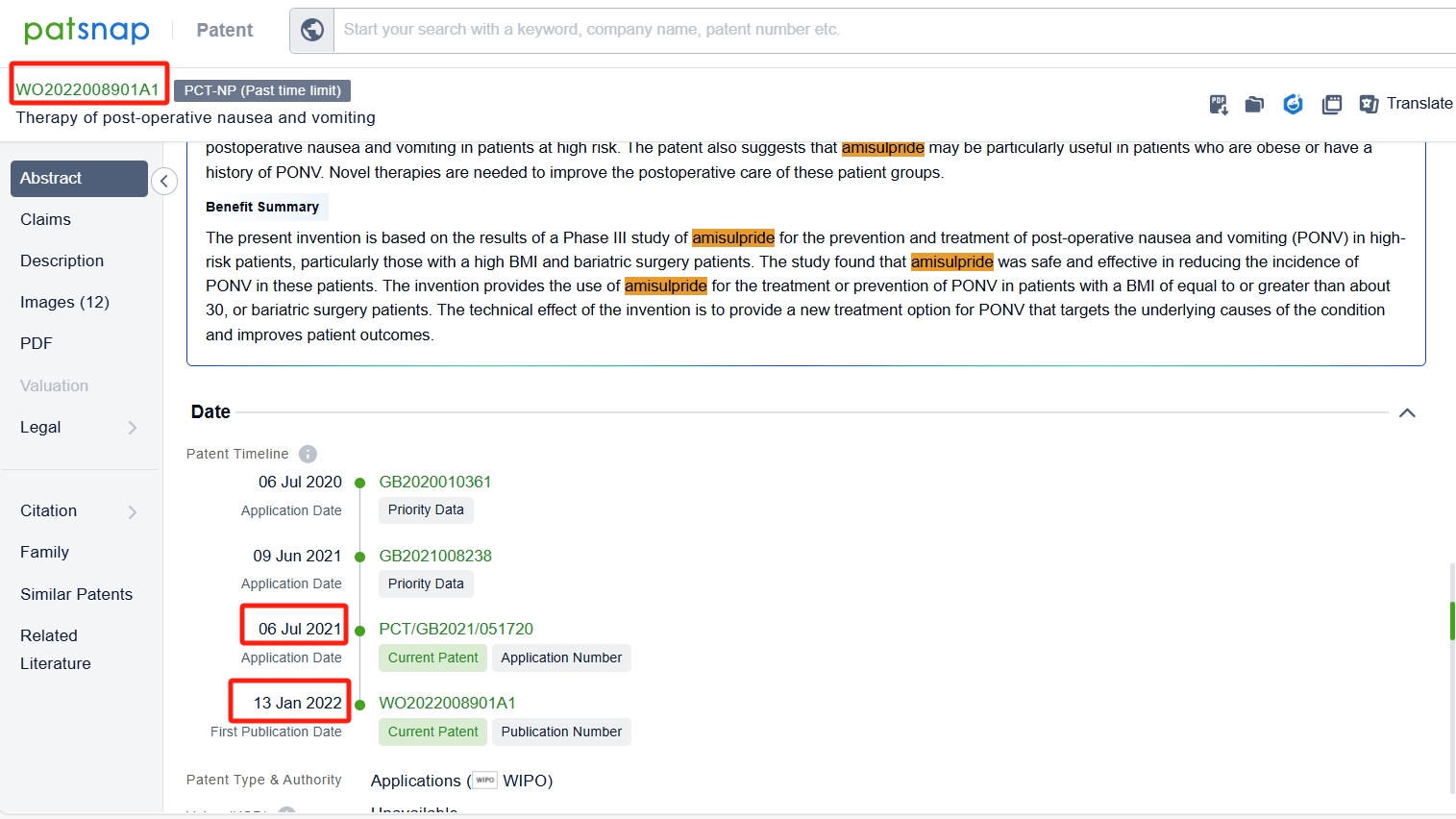
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Amisulpride. By reviewing the aforementioned patents, we can observe that Acacia Pharma Ltd.'s international patent WO2022008901A1 (application date 20210706, publication date 20220113) discloses a new use for Amisulpride in the treatment or prevention of Postoperative Nausea and Vomiting (PONV) in patients with a Body Mass Index (BMI) equal to or greater than approximately 30 or in patients undergoing weight loss surgery. Additionally, Saurashtra University's patent IN202121032772A (application date 20210721, publication date 20230123) is to provide a nano particulate nasal formulation of Amisulpride through intranasal administration which is safe effective and can be self-administered also, it is avoiding first pass metabolism because of nasal route of administration.
 Given the drug's approval status and target indications, it has the potential to significantly impact patient care in psychiatric and gastroenterological settings. The fact that it has attained approval in China, a significant market for pharmaceuticals, further underscores its relevance and potential commercial success.
Given the drug's approval status and target indications, it has the potential to significantly impact patient care in psychiatric and gastroenterological settings. The fact that it has attained approval in China, a significant market for pharmaceuticals, further underscores its relevance and potential commercial success.
Overall, Amisulpride is a well-established small molecule drug with approved indications for schizophrenia, postoperative nausea and vomiting, as well as nausea and vomiting. Its approval in China in 2004 marks a significant milestone, and its broad therapeutic potential suggests continued relevance and application in the field of biomedicine.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.





