Alector Shares AL002 Phase 2 Study Results and Company Updates
Alector, Inc. (Nasdaq: ALEC), a biotechnology firm at the clinical stage that is innovating unique, genetically validated treatments for neurodegenerative disorders, has shared findings from the Phase 2 INVOKE-2 clinical trial. This study assessed the safety and effectiveness of AL002 in mitigating disease progression among patients with early-stage Alzheimer's disease (AD). Administration of AL002 demonstrated prolonged target engagement and pharmacodynamic effects suggestive of microglial activation.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
AL002 did not succeed in achieving its main goal of slowing the clinical progression of Alzheimer's, as indicated by the Clinical Dementia Rating Sum of Boxes (CDR®-SB), and there were no treatment benefits observed for AL002 concerning secondary clinical and functional outcomes. Likewise, there were no significant benefits regarding Alzheimer’s fluid biomarkers for AL002, and amyloid PET imaging did not show any treatment-related decreases in brain amyloid levels. As previously noted, MRI results indicative of amyloid-related imaging abnormalities (ARIA) and infusion-related side effects were reported in the INVOKE-2 study, with ARIA occurrences mostly noted in participants receiving AL002.
“We at Alector understand the urgency of developing therapeutic options for Alzheimer’s disease and are dedicated to our goal of creating safe and effective treatments for the millions affected by neurodegenerative disorders globally,” stated Gary Romano, M.D., Ph.D., Chief Medical Officer at Alector. “With the comprehensive dataset from the INVOKE-2 trial, we intend to delve deeper into TREM2 biology. We are sincerely grateful to the committed investigators, patients, and caregivers who made this crucial trial feasible. We aim to present the trial findings to the scientific community soon, with the hope of enhancing the understanding of AD pathophysiology and advancing effective therapies for this devastating disease.”
Given the results, Alector has decided to discontinue the long-term extension study. The company continues to be dedicated to the development of its genetically validated and mechanically diverse drug candidates for treating neurodegenerative conditions. Central to this initiative are the programs aimed at increasing progranulin levels, including latozinemab and AL101/GSK4527226, which are being developed in partnership with GSK. Key findings from the INFRONT-3 Phase 3 clinical trial of latozinemab for frontotemporal dementia linked to a progranulin gene mutation are anticipated to be available in late 2025 or early 2026. The PROGRESS-AD global Phase 2 clinical study, assessing AL101/GSK4527226 in early Alzheimer’s, has surpassed one-third of its goal of enrolling 282 participants. Additionally, Alector is making progress with its preclinical candidates targeting a variety of protein and enzyme targets.
Beyond advancing its product pipeline, the company is also focusing on enhancing its proprietary and adaptable blood-brain barrier technology platform, Alector Brain Carrier (ABC). The goal of ABC is to improve the delivery of therapeutic antibodies, proteins, and enzymes, achieve greater penetration and effectiveness at lower doses, and ultimately enhance patient outcomes while decreasing costs.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
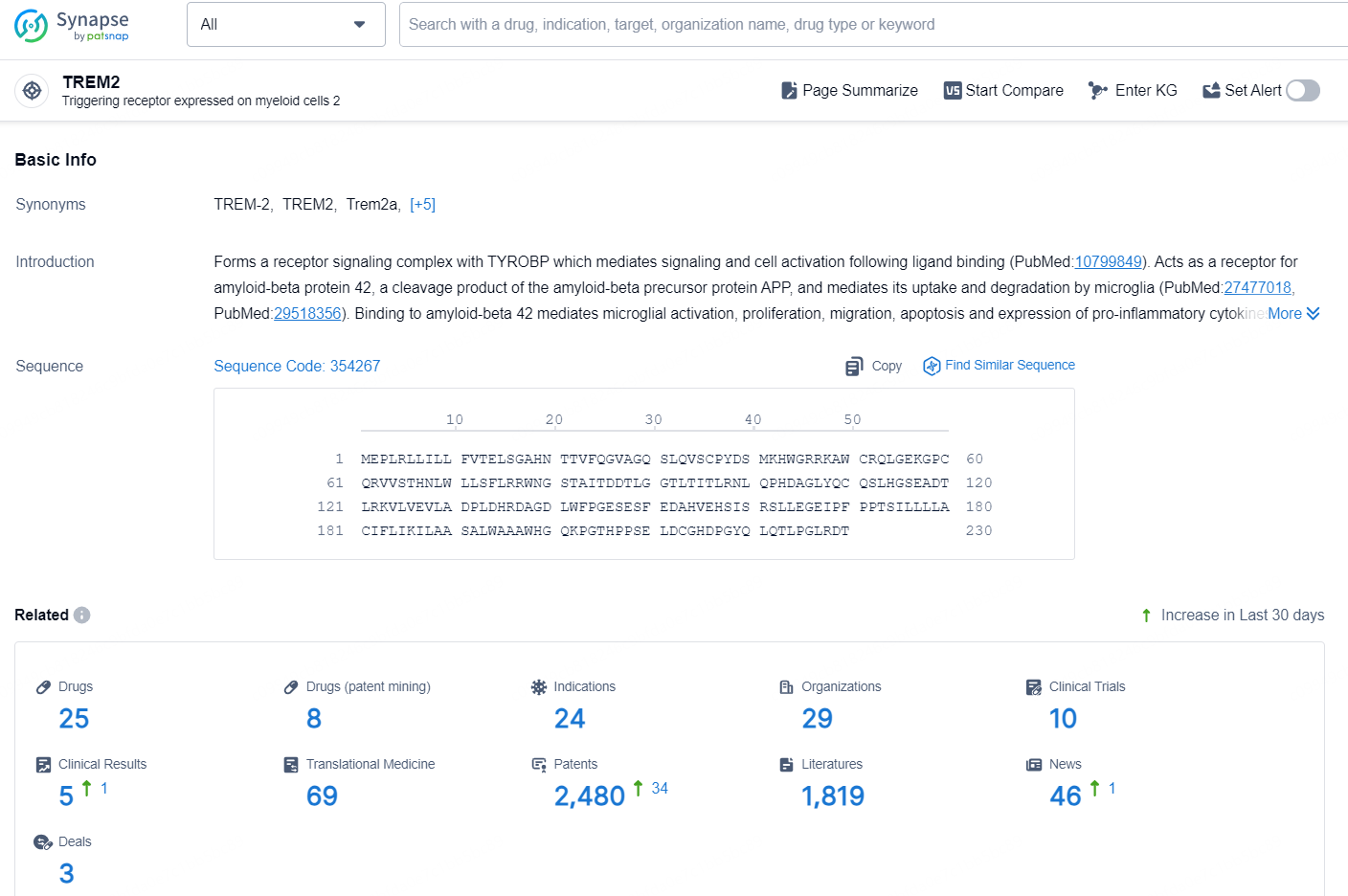
According to the data provided by the Synapse Chemical, As of December 4, 2024, there are 25 investigational drugs for the TREM2 target, including 24 indications, 29 R&D institutions involved, with related clinical trials reaching 10, and as many as 2480 patents.
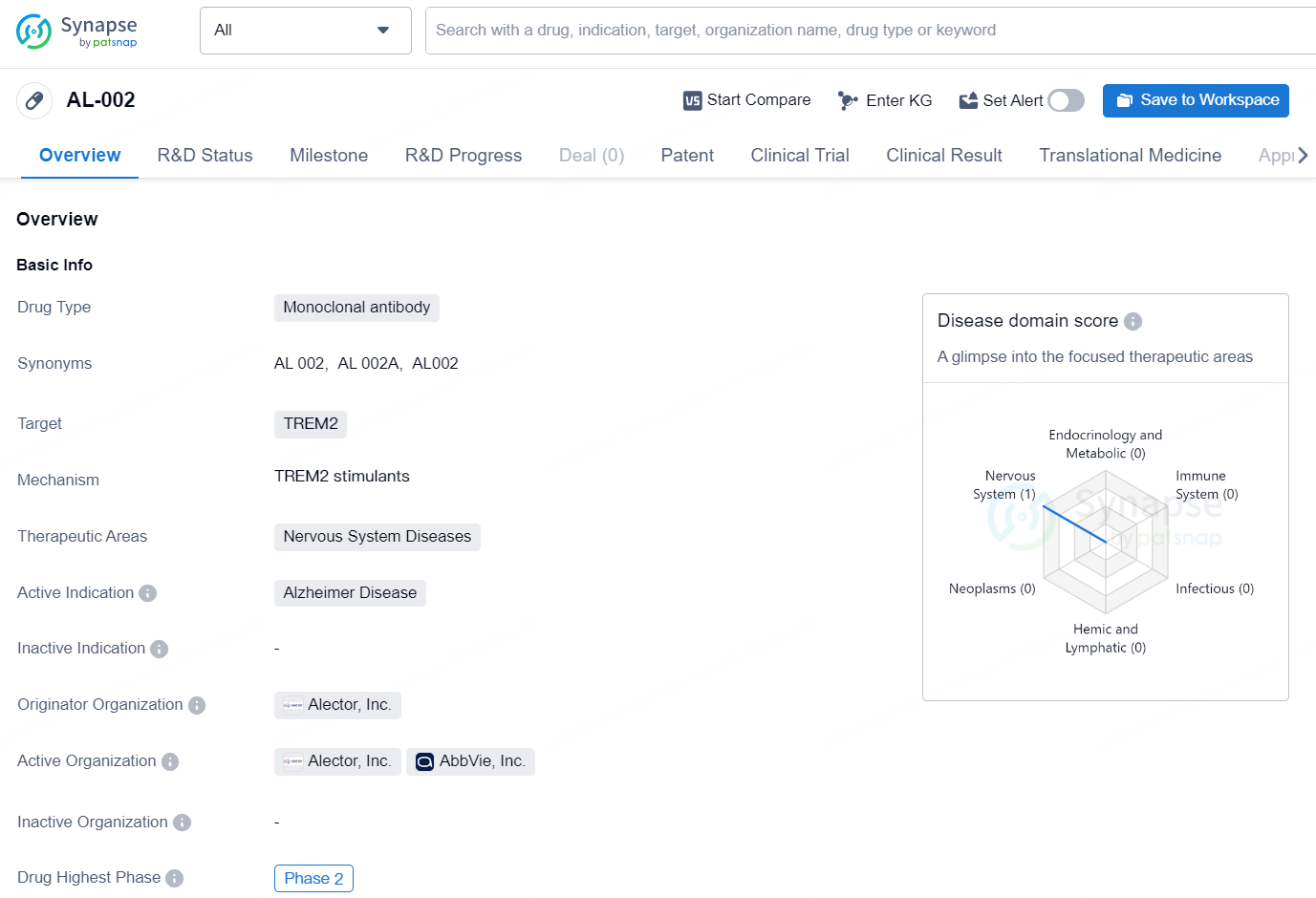
AL-002 is a monoclonal antibody drug that targets the protein TREM2. It falls under the therapeutic area of Nervous System Diseases with an active indication for Alzheimer Disease. The drug is being developed by Alector, Inc., an organization known for its focus on developing immunotherapies for neurodegenerative disorders.