Tenaya Begins Phase 1b Trial of TN-401 for ARRVC Linked to PKP2
Tenaya Therapeutics, Inc. (NASDAQ: TNYA), a biotechnology firm currently in the clinical phases, is dedicated to identifying, developing, and providing potential cures targeting the fundamental causes of heart disease. The company has revealed that the inaugural patient has received TN-401 gene therapy in the RIDGE-1 Phase 1b clinical study at the University of California, San Francisco. Tenaya expects to present preliminary results from the RIDGE-1 trial in the year 2025.
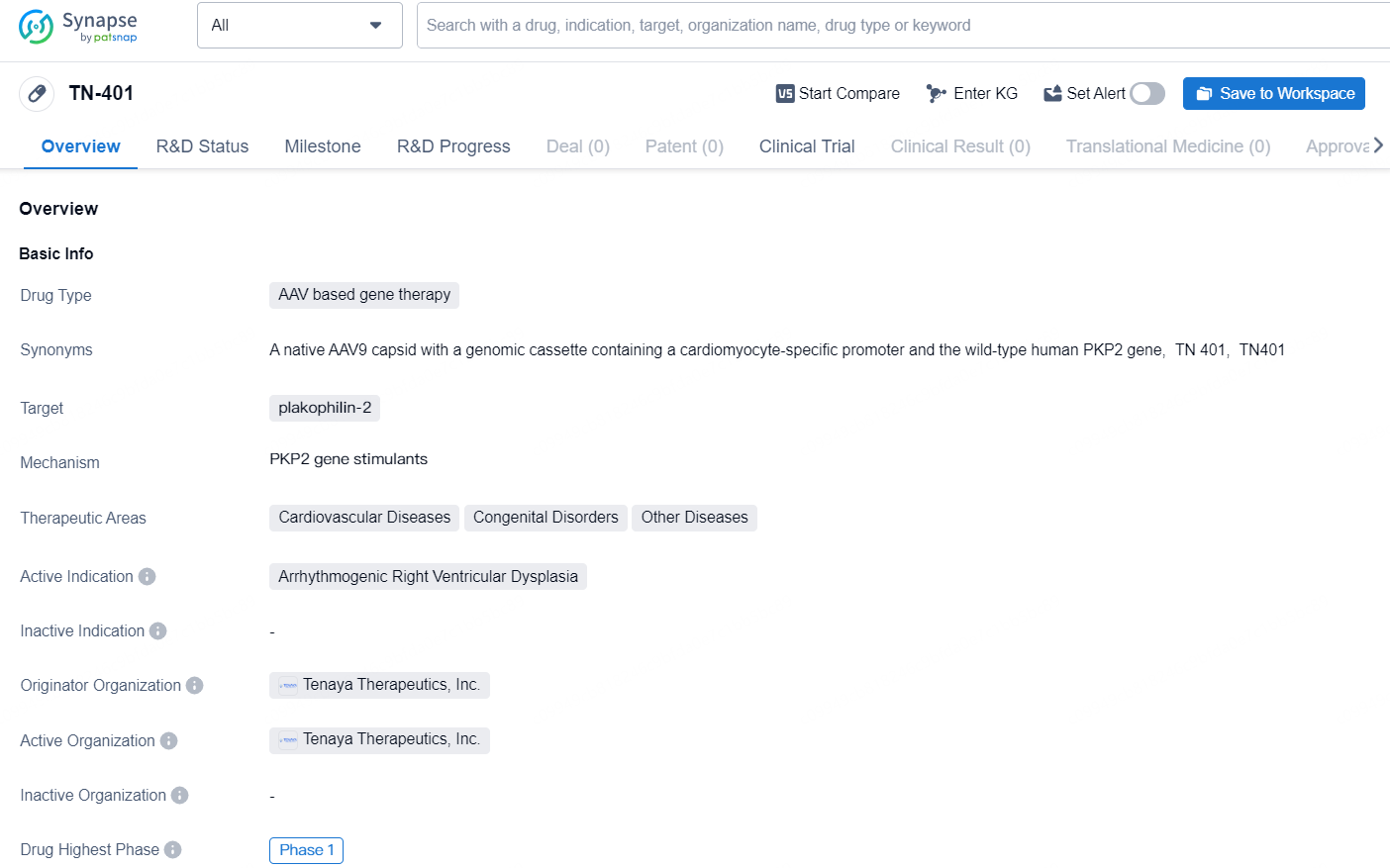
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
TN-401 is undergoing development as a treatment for arrhythmogenic right ventricular cardiomyopathy (ARVC), also recognized as arrhythmogenic cardiomyopathy (ACM), stemming from mutations in the plakophilin-2 (PKP2) gene. Mutations within the PKP2 gene lead to inadequate levels of vital proteins necessary for maintaining the structural stability and intercellular communication in cardiac muscle cells.
The TN-401 gene replacement therapy aims to introduce a functional PKP2 gene into cardiac cells utilizing an adeno-associated virus serotype 9 (AAV9) vector. In preclinical investigations, the healthy PKP2 gene was effectively integrated into heart cells, resulting in the production of the previously missing protein, thereby potentially slowing or even reversing disease progression. In comparisons with untreated in vivo knockout models, treatment with TN-401 resulted in normalized heart rhythms, halted disease advancement, and prolonged survival after a single treatment.
“Individuals affected by ARVC often face life-threatening arrhythmias and have a heightened risk of developing heart failure, experiencing cardiac arrest, or sudden death. To mitigate these risks, ARVC patients must endure substantial lifestyle limitations, rely on long-term medications, and undergo various interventions, all of which adversely affect their quality of life without directly addressing the root cause of their genetic defect,” explained Vasanth Vedantham, M.D., Ph.D., a Professor of Medicine and Cardiac Electrophysiologist, as well as the Director of Cardiovascular Genetics at the University of California, San Francisco. He is also an investigator involved in the RIDGE-1 Phase 1b clinical trial. “Mutations in the PKP2 gene represent the predominant single-gene cause of ARVC, and unlike current therapies for ARVC, TN-401 aims to tackle the fundamental issue of the disease by delivering a fully functional PKP2 gene to the heart.”
The RIDGE-1 Phase 1b clinical trial is a multi-center, open-label, dose escalation study taking place in the U.S. and the UK. This trial aims to evaluate the safety, tolerability, and initial clinical effectiveness of a single intravenous infusion of TN-401. Efforts will be made to enroll up to fifteen adults diagnosed with PKP2-associated ARVC who have an implantable cardioverter defibrillator (ICD) and are identified as being at an elevated risk for arrhythmias based on the count of premature ventricular contractions during the screening process.
The first dose of TN-401 to be administered in the RIDGE-1 clinical trial is 3E13 vg/kg, which demonstrated near-maximal efficacy in prior preclinical evaluations. The initial three patients will receive the treatment in a sequential manner. After three patients have been dosed at the 3E13 vg/kg level, an independent safety review panel will provide recommendations on further dose escalation and/or the expansion of participant enrollment for the initial cohort.
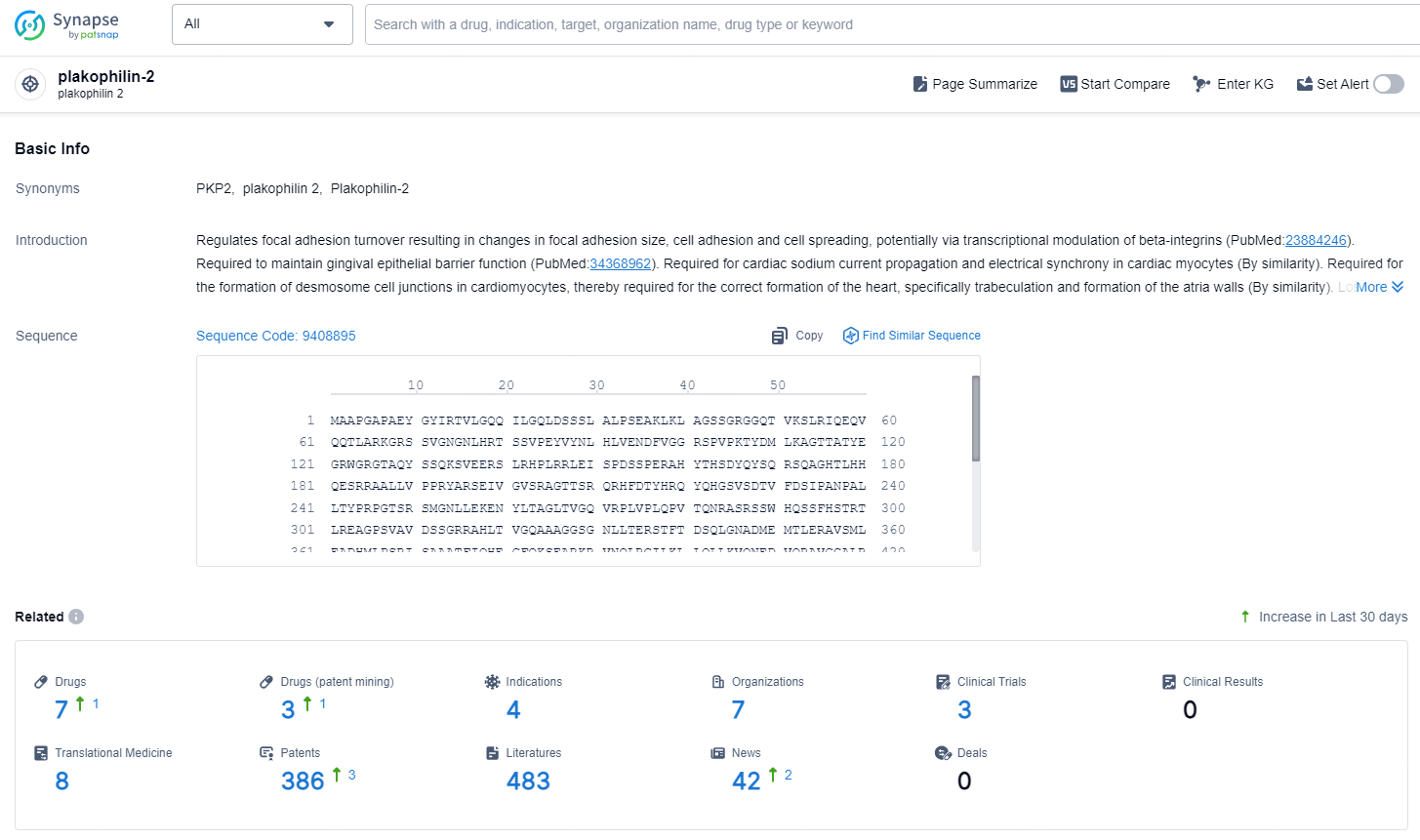
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 4, 2024, there are 7 investigational drugs for the plakophilin-2 target, including 4 indications, 7 R&D institutions involved, with related clinical trials reaching 3, and as many as 386 patents.
TN-401 is an AAV based gene therapy drug developed by Tenaya Therapeutics, Inc. The drug aims to target plakophilin-2 and is primarily focused on treating Arrhythmogenic Right Ventricular Dysplasia, a cardiovascular disease. However, the therapeutic scope of TN-401 also encompasses congenital disorders and other diseases within the cardiovascular field.