Alentis Therapeutics Secures FDA IND Approval for Innovative CLDN1-ADC, ALE.P02
Alentis Therapeutics, a biotechnology company at the clinical development stage focusing on treatments for Claudin-1 positive (CLDN1+) cancers and organ fibrosis, revealed that the U.S. Food & Drug Administration (FDA) has approved an IND application for ALE.P02. This substance is an anti-CLDN1 antibody-drug conjugate (ADC) that incorporates a tubulin inhibitor as its payload. A clinical trial of Phase 1/2 is anticipated to commence in the first quarter of 2025 targeting patients with CLDN1+ squamous cell tumors.
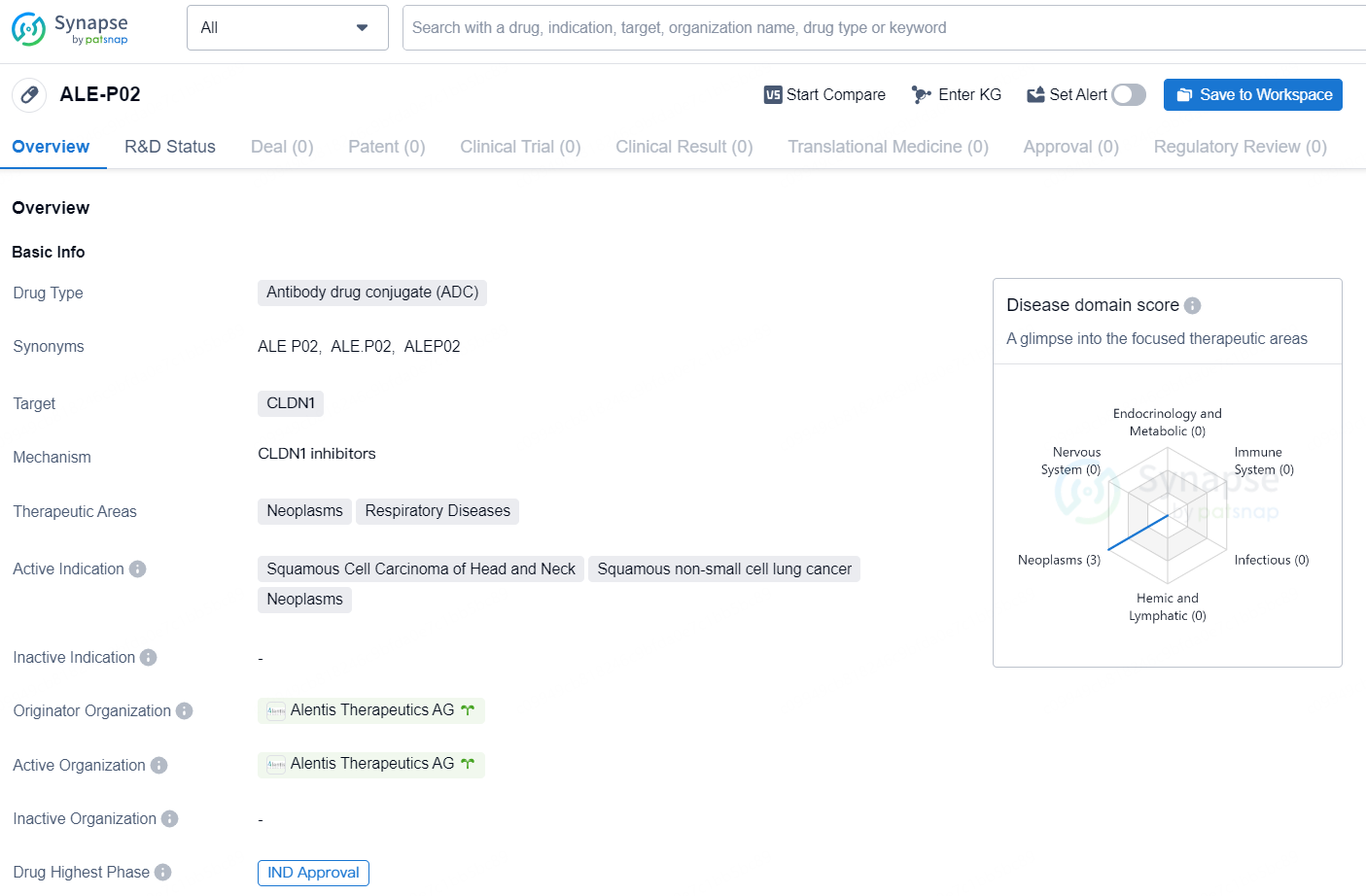
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"ADC therapies have demonstrated significant promise in cancer treatment," stated Luigi Manenti, who serves as the Chief Medical Officer at Alentis. "Cancers such as those of the head and neck, cervix, esophagus, and lung, which originate from squamous cells, typically exhibit high levels of CLDN1 expression. ALE.P02 represents a novel opportunity for patients requiring new treatment options following the failure of first-line therapies."
"Targeting CLDN1 with ADCs is captivating due to their potential to meet the urgent demand for new targets within the ADC field," remarked Tony Mok, a Clinical Oncology Professor at the Chinese University of Hong Kong. "ALE.P02 holds particular promise for squamous cell cancers, including HNSCC and NSCLC, where CLDN1 is frequently overexpressed. The medical needs in these cases are considerable, and I am eager to see the outcomes of the Phase 1/2 study."
According to Dr. Roberto Iacone, CEO of Alentis, "The initiation of clinical trials for ALE.P02 represents a meaningful progression in our oncology portfolio. By utilizing data from human trials involving lixudebart (ALE.F02), which is used as the fundamental antibody of Alentis' ADCs, we can optimize our development strategy."
Dr. Iacone further mentioned, "For our second ADC initiative, ALE.P03, featuring a topoisomerase I inhibitor payload, we intend to start a first-in-human clinical trial in 2025."
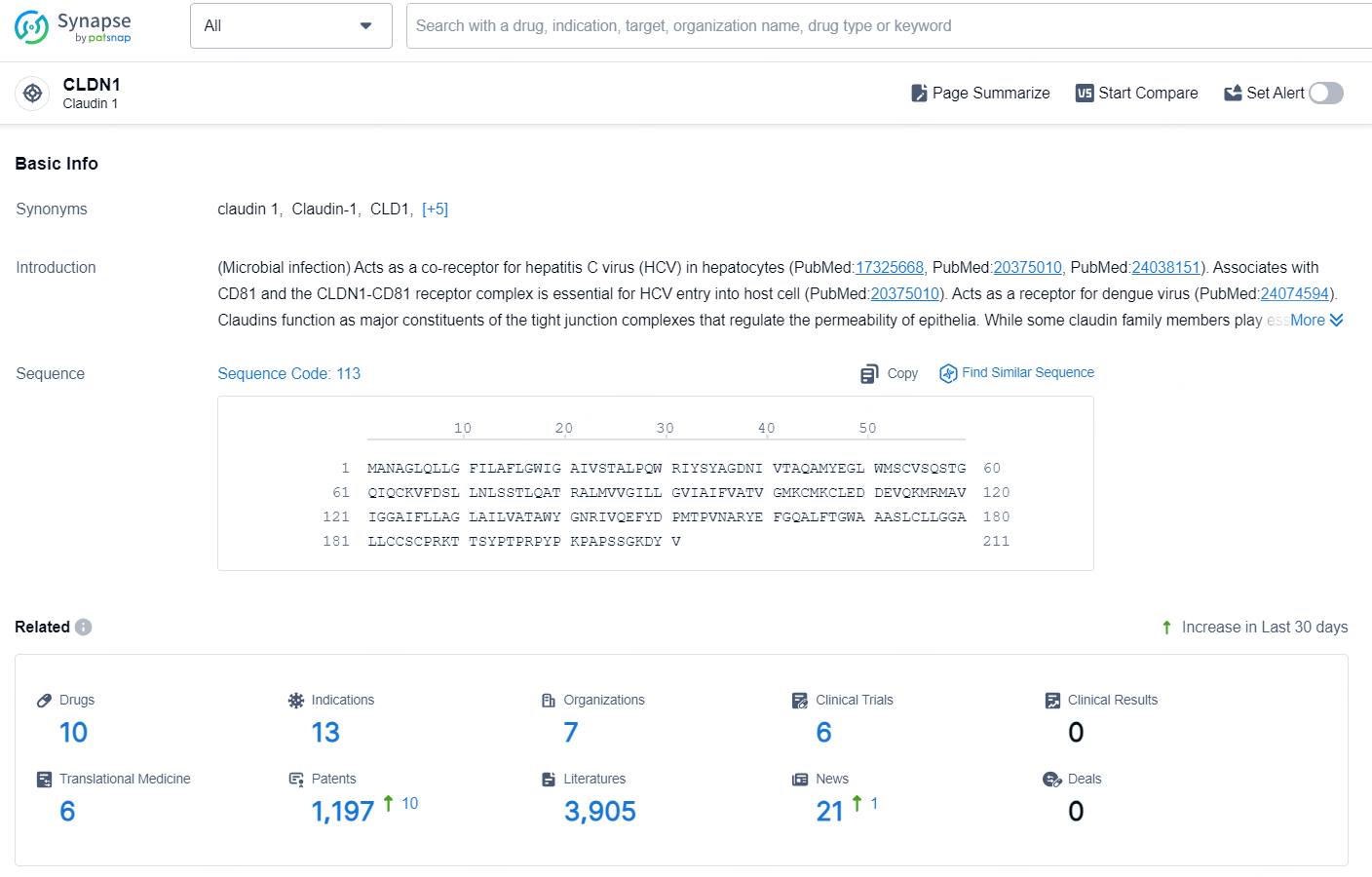
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 9, 2024, there are 10 investigational drug for the CLDN1 target, including 13 indications, 7 R&D institutions involved, with related clinical trials reaching 6, and as many as 1197 patents.
ALE-P02 is an antibody drug conjugate (ADC) that targets CLDN1, a tight junction protein often overexpressed in various types of cancer. The drug is being developed to treat neoplasms and respiratory diseases, with its active indications including squamous cell carcinoma of the head and neck, squamous non-small cell lung cancer, and neoplasms.