Allyx Therapeutics Reports Initial Parkinson’s Patient Treated with ALX-001
Allyx Therapeutics has reported the administration of its primary compound, ALX-001, to the initial patient in a fresh clinical trial evaluating safety, pharmacokinetics, and possible therapeutic effects in individuals with Parkinson’s disease. ALX-001 is a uniquely selective, pioneering, synapse-focused, disease-altering oral medication. Allyx Therapeutics operates as a clinical-stage biotech firm aiming to introduce an innovative strategy to maintain and safeguard synapses for those affected by neurodegenerative disorders.
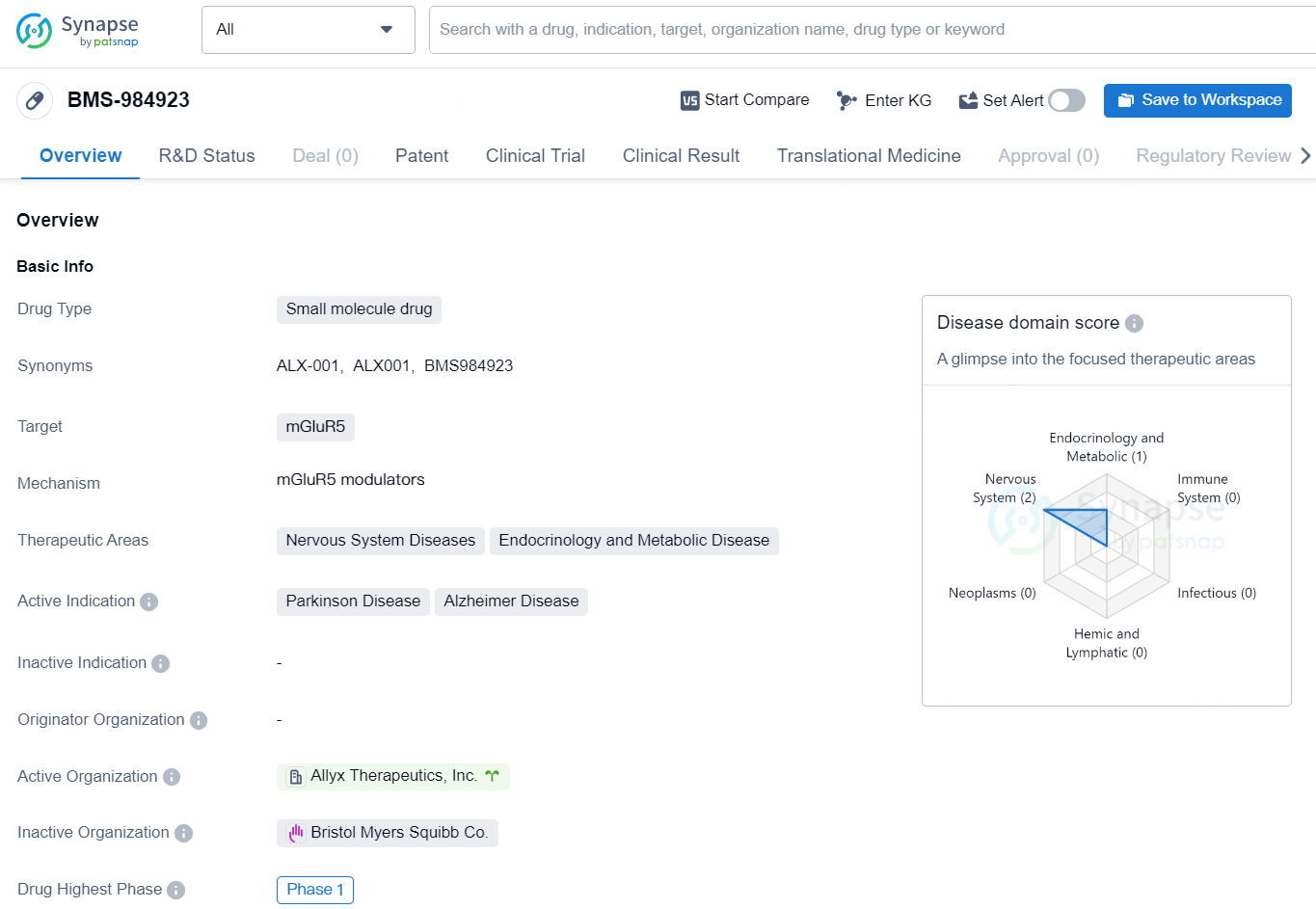
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Allyx Therapeutics is advancing with significant momentum to develop ALX-001 as the inaugural disease-modifying small molecule for neurodegenerative disorders. This development includes two simultaneous safety trials involving patients with Parkinson’s disease and Alzheimer’s disease, contributing vital data to the scholarly understanding of this innovative treatment methodology," stated Tim Siegert, Ph.D., chief operating officer and co-founder of Allyx Therapeutics.
The safety of ALX-001 is being evaluated in a 28-day trial for Parkinson’s disease (NCT06309147), where adults aged between 21 and 80 years are administered either 50 mg or 100 mg of ALX-001 twice daily, or a placebo. This study also examines dopamine transporter levels in the brain via single photon emission computed tomography to identify early therapeutic responses related to synapse restoration. The Duke Clinical Research Institute (DCRI) is leading this trial with funding support from The Michael J. Fox Foundation for Parkinson’s Research.
"I am excited to work with the Allyx team to further explore how Parkinson’s disease impacts the brain and to assess potential impactful treatment options for patients. Investigating the safety of this potentially disease-modifying small molecule is a vital initial phase, and I am pleased to contribute to this effort," remarked Laurie H. Sanders, Ph.D., associate professor of Neurology at Duke University School of Medicine and DCRI.
Building on over twelve years of clinical investigation, ALX-001 has shown continual potential in current studies. The ALX-001 initiative has garnered over $20 million in grant funding from the National Institutes of Health, the U.S. Government’s Small Business Innovation Research (SBIR) programs, the Alzheimer's Association, and The Michael J. Fox Foundation for Parkinson’s Research, among others.
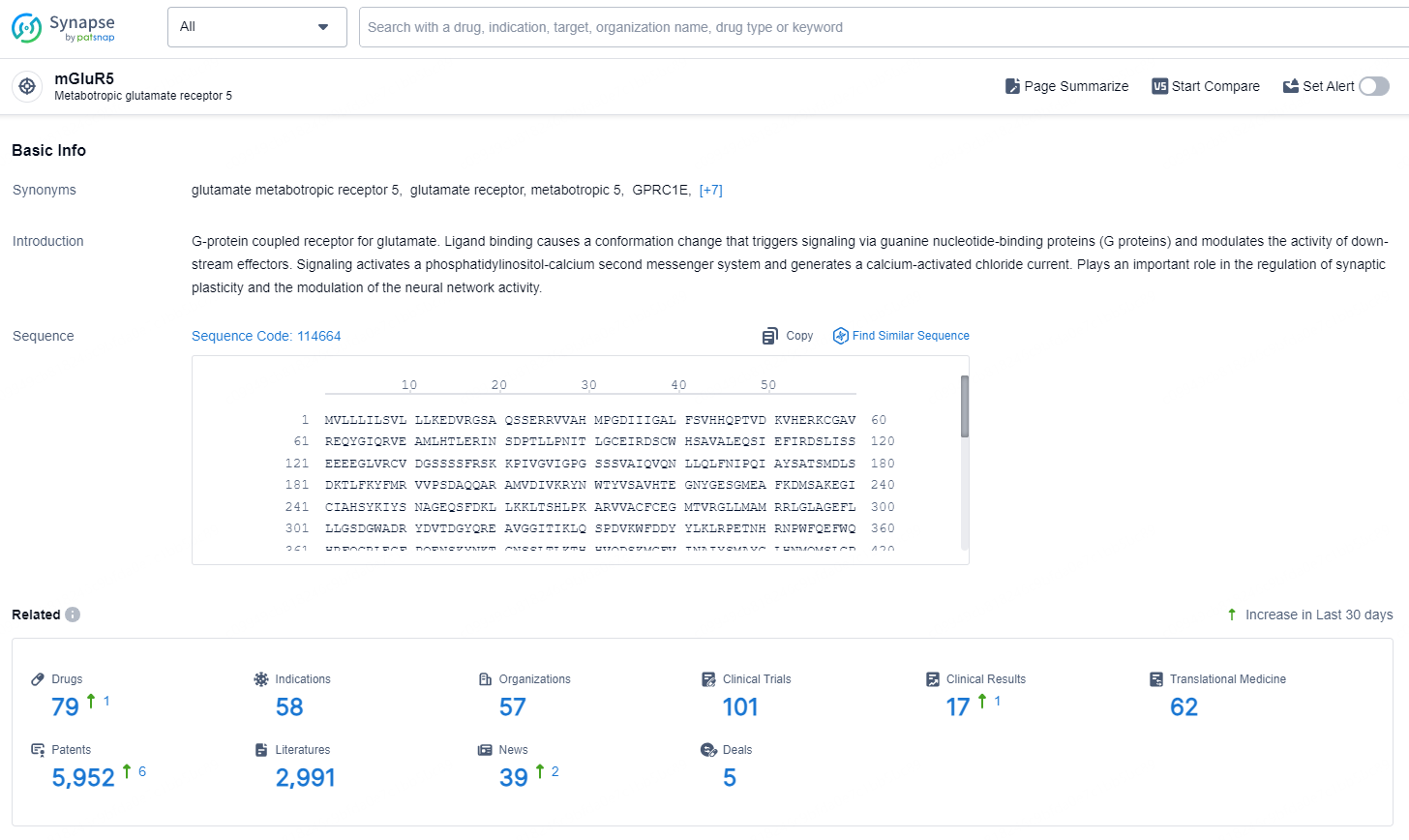
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 455 investigational drugs for the mGluR5 targets, including 879 indications, 467 R&D institutions involved, with related clinical trials reaching 8291, and as many as 82197 patents.
ALX-001 (previously BMS-984923) is a silent allosteric modulator of mGluR5, and is a first-in-class compound that selectively blocks the pathogenic activation of the receptor while preserving the normal physiological glutamate signaling that is required for cognition. As such, ALX-001 has a wide therapeutic window that can saturate receptors while avoiding on-target toxicity observed with negative allosteric modulators. mGluR5 has been shown to be essential for mediating synaptic dysfunction and loss caused by multiple misfolded extracellular protein species, and as such, presents a novel approach for treating Alzheimer’s and Parkinson’s disease.