Alnylam Unveils Phase 3 HELIOS-B Study Results of Vutrisiran in ATTR Cardiomyopathy at ESC Congress
Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY), a prominent RNAi therapeutics firm, revealed comprehensive findings from the HELIOS-B Phase 3 clinical trial evaluating vutrisiran. Vutrisiran is an investigational RNAi treatment being developed for addressing ATTR amyloidosis with cardiomyopathy (ATTR-CM).
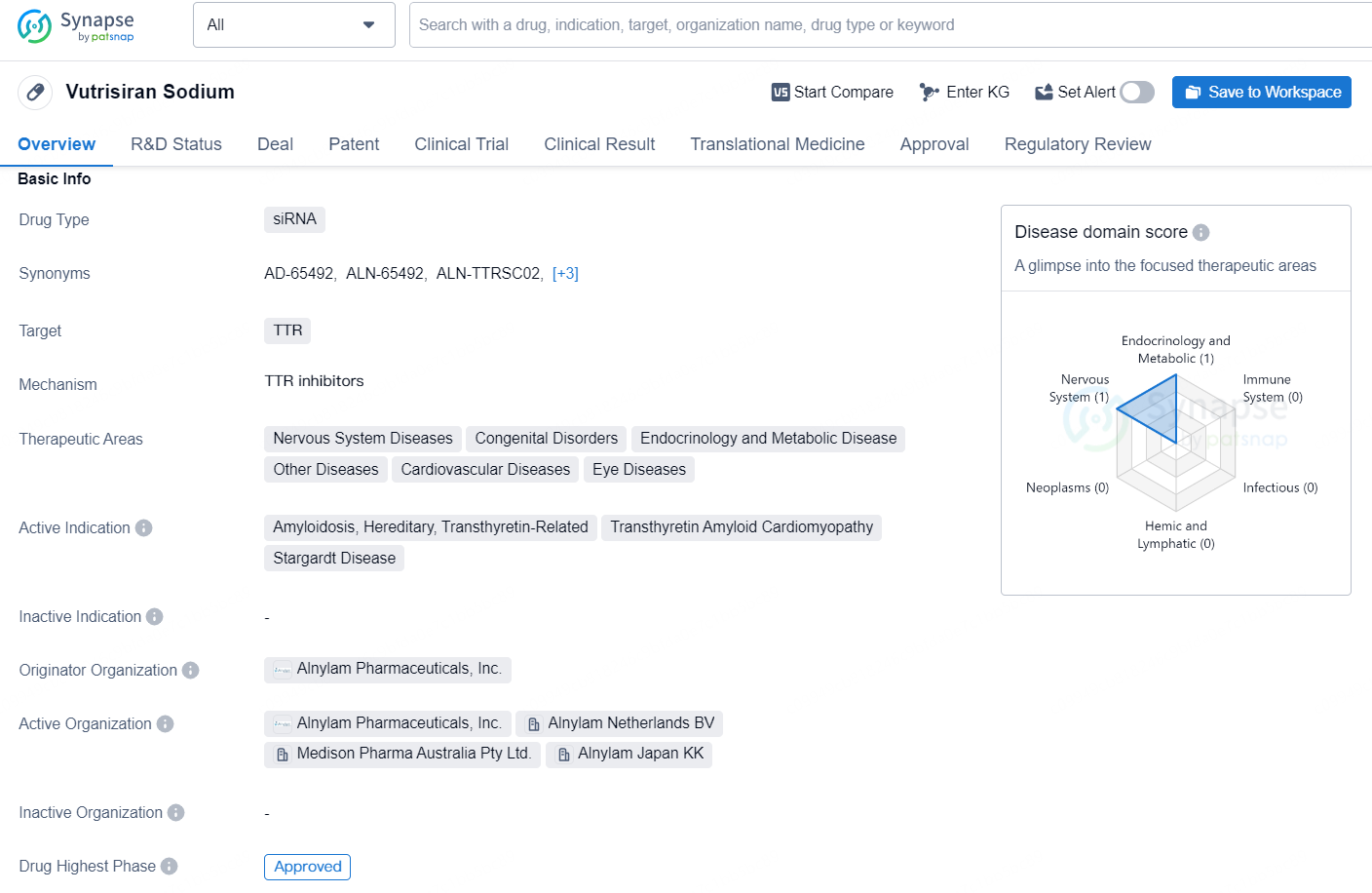
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data were unveiled today during a Hot Line session at the European Society of Cardiology (ESC) Congress 2024, held from August 30 to September 2 in London, United Kingdom. Concurrently, findings from the HELIOS-B study were published in The New England Journal of Medicine.
As previously stated, the HELIOS-B study successfully achieved all 10 primary and secondary endpoints with statistical significance in both the overall and monotherapy cohorts.
Participants were primarily New York Heart Association (NYHA) Class I or II with wild-type disease, diagnosed through non-invasive techniques, and were undergoing significant concurrent therapies such as tafamidis and SGLT2 inhibitors, indicative of the current ATTR-CM patient demographic.
The study showed that treatment with vutrisiran markedly decreased the risk of death and cardiovascular incidents compared to placebo (refer to the table below for specifics). In the overall cohort, vutrisiran lowered the risk of all-cause mortality and recurrent cardiovascular events by 28%, with comparable reductions in both mortality and cardiovascular events as components of the endpoint. Mortality in this group was substantially reduced by 31% during the double-blind phase and by 36% up to 42 months. In the monotherapy group, vutrisiran notably lowered the risk of all-cause mortality and recurrent cardiovascular events by 33% and significantly decreased the risk of mortality by 35% up to 42 months. In the monotherapy group during the double-blind period, a non-significant 30% reduction in mortality was observed (nominal p-value 0.1179) as a component of the primary endpoint.
Vutrisiran also demonstrated benefits over placebo across several well-established clinical measures of disease progression, including the 6-Minute Walk Test, Kansas City Cardiovascular Questionnaire, and NYHA Class, as well as the cardiac biomarker NT-proBNP.
Subgroup analyses highlighted consistent benefits across all key patient segments, including those on background tafamidis. Greater efficacy trends were noted in patients with early-stage disease (e.g., younger patients and those with lower baseline NT-proBNP).
In HELIOS-B, the safety and tolerability profiles of vutrisiran were consistent with those seen in the approved patient population and in earlier clinical studies.
“The results from the HELIOS-B study represent a significant advancement in the treatment of ATTR amyloidosis with cardiomyopathy, indicating that TTR production knockdown with vutrisiran can significantly lower all-cause mortality and cardiovascular events,” remarked Marianna Fontana, M.D., Ph.D., HELIOS-B investigator and Professor of Cardiology at University College London, National Amyloidosis Center, Royal Free Hospital, London. “Over the past decade, advancements in ATTR-CM have led to earlier diagnoses, often with milder symptoms and better prognosis, as well as more comprehensive background standards of care. In this modern context, the benchmark for demonstrating benefits was high. These HELIOS-B findings also imply that vutrisiran may offer greater advantages for patients in the earlier stages of the disease, where the progressive nature of ATTR-CM makes early intervention crucial for preserving functional capacity and quality of life.”
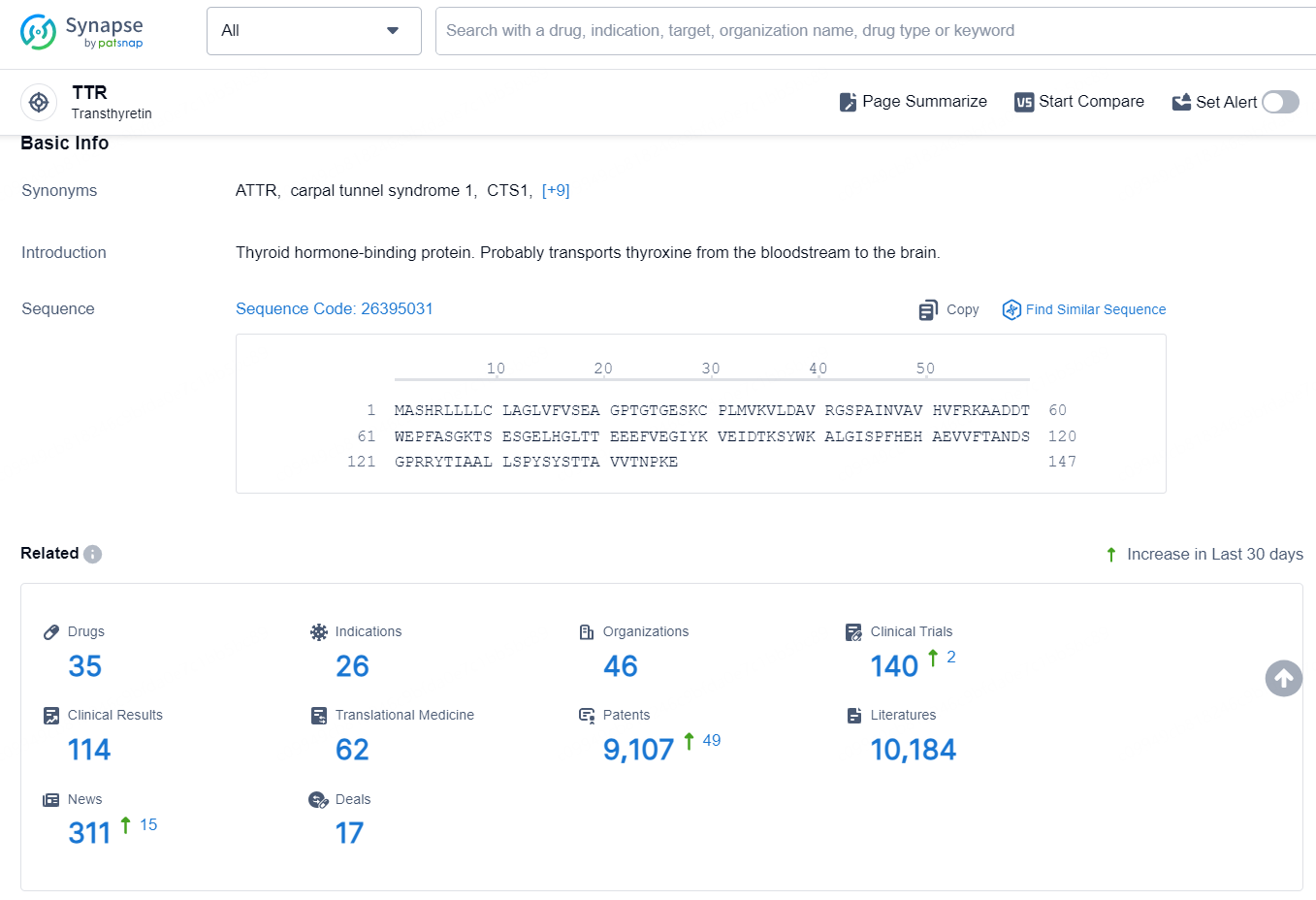
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 3, 2024, there are 23 investigational drugs for the TTR targets, including 37 indications, 59 R&D institutions involved, with related clinical trials reaching 215, and as many as 10772 patents.
AMVUTTRA® (vutrisiran) is an RNAi therapeutic that delivers rapid knockdown of mutant and wild‑type transthyretin (TTR), addressing the underlying cause of transthyretin (ATTR) amyloidosis. Administered quarterly via subcutaneous injection, vutrisiran is approved and marketed in more than 15 countries for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR-PN) in adults. In the UK, vutrisiran is specifically indicated for the treatment of hATTR in adult patients with stage 1 or stage 2 polyneuropathy. Vutrisiran is also in development for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM), which encompasses both wild-type and hereditary forms of the disease.