Alnylam's KARDIA-2 Trial: Positive Zilebesiran Results in Uncontrolled Hypertension
Alnylam Pharmaceuticals, Inc., a pioneer in RNA interference (RNAi) treatment development, has disclosed encouraging findings from their KARDIA-2 Phase 2 clinical trial, which assessed the benefits and safety profile of zilebesiran via a one-time subcutaneous administration in conjunction with one of the customary antihypertensive therapies. The treatments used in the study were a thiazide analog diuretic (indapamide), a calcium channel antagonist (amlodipine), and an angiotensin II receptor antagonist (olmesartan).
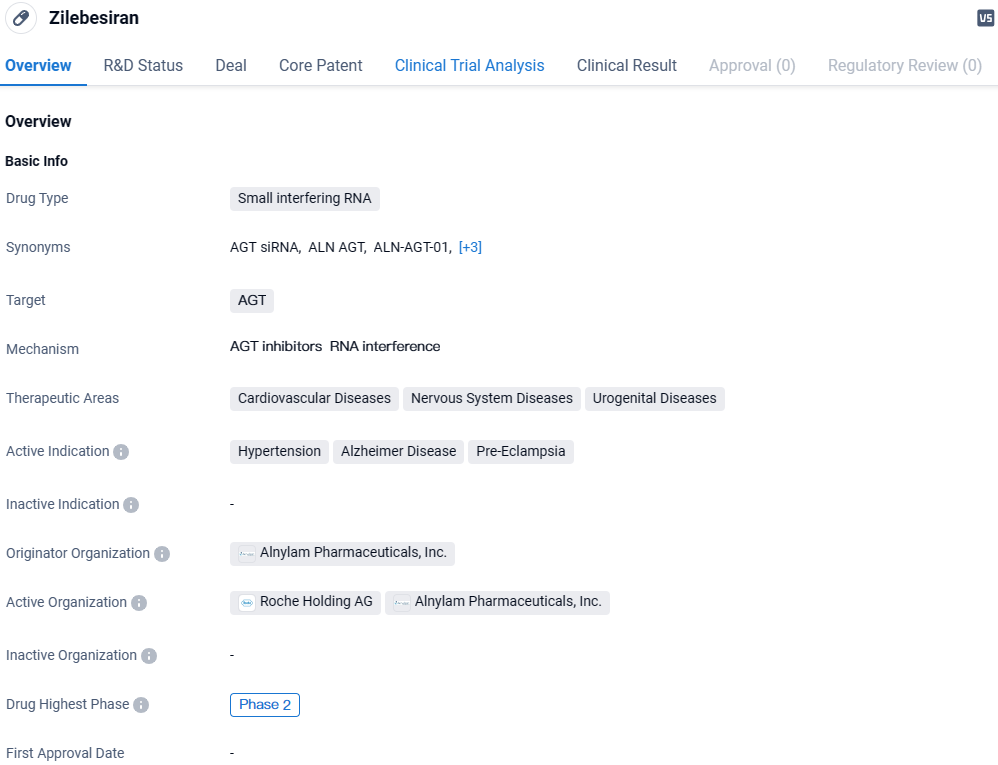
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Zilebesiran, an RNAi agent under exploration aimed at AGT produced by the liver, is in the research phase for potentially managing high blood pressure by potentially requiring only two doses yearly. Today's session at the American College of Cardiology Annual Scientific Session for 2024 included a presentation of this groundbreaking clinical trial. Earlier in March 2024, the Company shared promising preliminary findings from the pivotal KARDIA-2 trial.
In KARDIA-2, the main goal was reached, presenting both clinically meaningful and statistically substantial additional reductions in the average systolic blood pressure over 24 hours—factoring out placebo effects—of as much as 12.1 mmHg. This measurement was established through ambulatory BP monitoring after the combination of zilebesiran with a thiazide-like diuretic, a calcium channel blocker, or an angiotensin receptor blocker, confirmed separately at the 3-month mark. The crucial secondary goal focused on a notable decrease in clinicians' assessments of SBP at offices for all three distinct drug cohorts at Month 3.
By the half-year mark, zilebesiran continued to show significant and prolonged reductions in office SBP after adjustments for placebo, with respect to time, upon addition to indapamide, amlodipine, and olmesartan, even with the introduction of supplementary antihypertensive remedies post Month 3.
Moreover, when zilebesiran was used in combination with indapamide and amlodipine, there were significant, time-adjusted decreases in the average 24-hour SBP, gauged by ABPM, which persisted until the 6-month interval. However, when zilebesiran was added to the peak dosage of olmesartan, the results perceived at the 6-month juncture for the average 24-hour SBP adjustment from the baseline, measured through ABPM, did not achieve statistical significance.
Dr. Akshay Desai, responsible for the Cardiomyopathy and Heart Failure Program at Brigham and Women's Hospital, remarked: "Zilebesiran could potentially overcome numerous shortcomings associated with existing hypertension therapies. While more data is needed to validate its persisting efficiency and safety in a wider patient pool, the preliminary results are promising. The idea of regulating blood pressure effectively with just two yearly injections could revolutionize patient care."
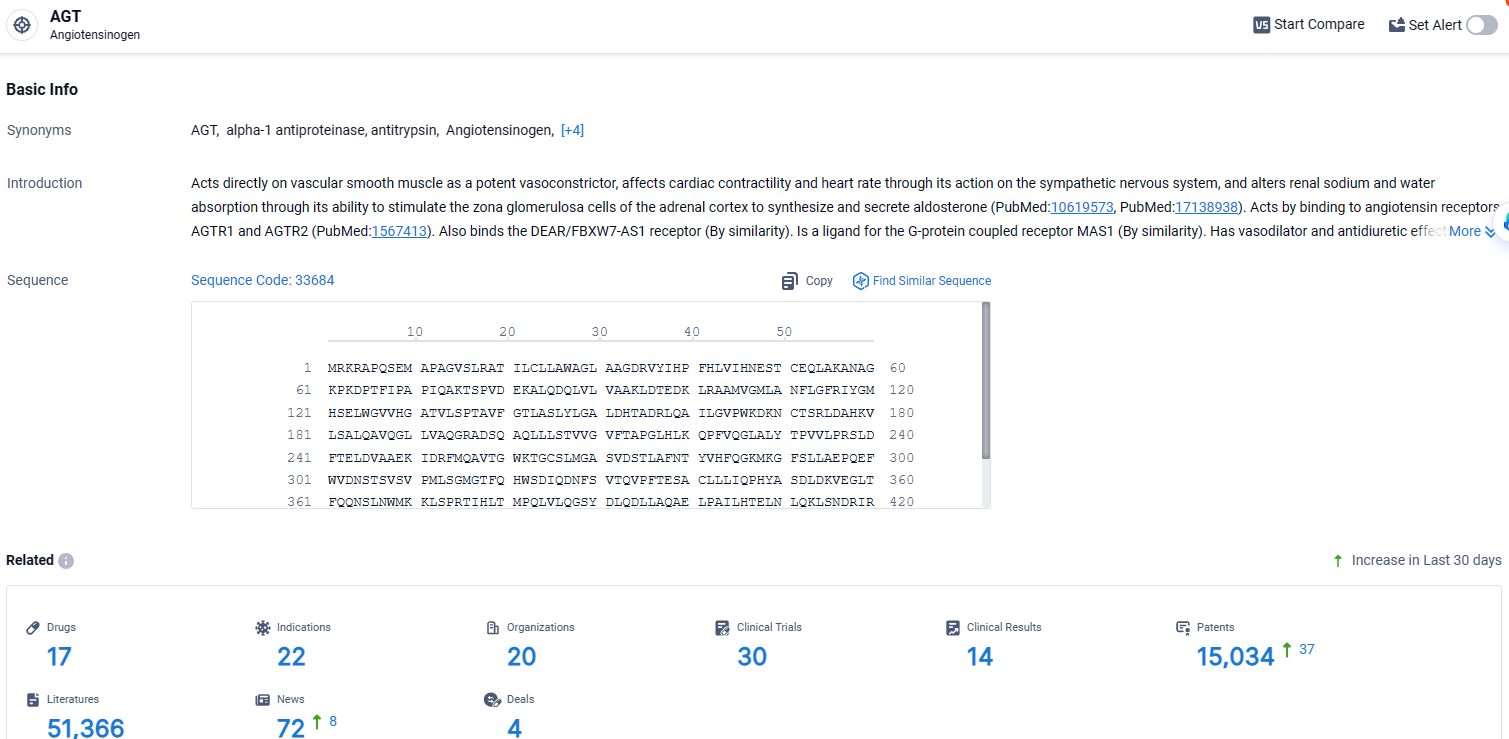
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 8, 2024, there are 17 investigational drugs for the AGT target, including 22 indications, 20 R&D institutions involved, with related clinical trials reaching 30, and as many as 15034 patents.
Zilebesiran targets AGT and is being investigated for its potential therapeutic applications in cardiovascular diseases, nervous system diseases, and urogenital diseases. The drug is currently in Phase 2 of development and shows promise in treating hypertension, Alzheimer's disease, and pre-eclampsia. Further research and clinical trials will be necessary to determine the drug's ultimate efficacy and safety profile.