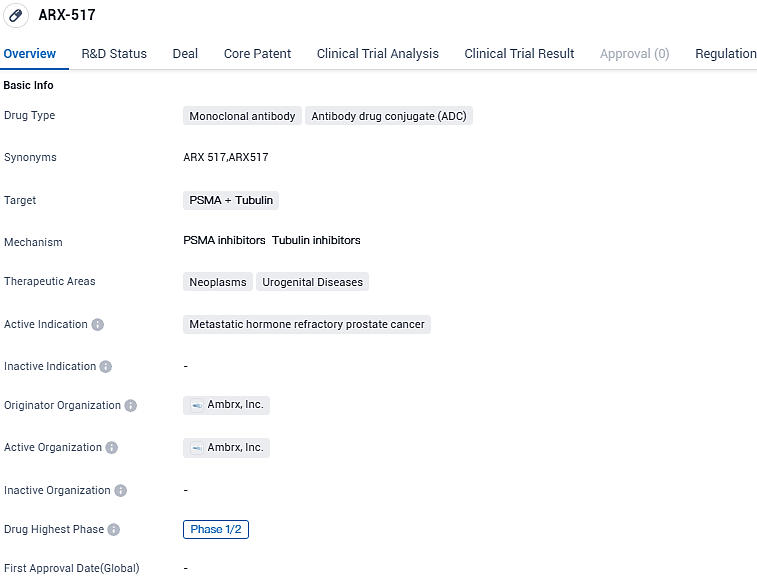
Ambrx Reports Advances in APEX-01, Phase 1/2 Trial of ARX517, a PSMA-Targeted ADC, in Hormone-Refractory Advanced Prostate Cancer
Ambrx Biopharma, Inc. has released a new clinical progress report pertaining to their unique anti-PSMA antibody-drug conjugate, ARX517. This report relates to the APEX-01 Phase 1/2 clinical trial, which is currently expanding and escalating doses for patients with advanced metastatic castration-resistant prostate cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
After monitoring the safety profile for a period of 21 days following the administration of the APEX-01 trial's dose-escalation group, Cohort 9, which received a dosage of 3.4 mg/kg, there were no instances of dose-limiting toxicities (DLTs) or serious adverse events (SAEs). In this cohort, two patients exhibited a significant drop in prostate-specific antigen (PSA) levels by 91% and 33%, respectively, at their initial assessment three weeks after receiving just one dose of ARX517.
The third participant from the same group did not show a decline in PSA levels due to their specific type of metastatic castration-resistant prostate cancer (mCRPC), which doesn't produce PSA. On November 27, which was a Monday, the committee responsible for overseeing safety unanimously agreed to proceed with a higher dose of 4.5 mg/kg while also opting to continue enrolling subjects at the dose of 3.4 mg/kg.
Each of the previously established expansion cohorts has reached its full capacity; this includes Cohort 4 (1.4 mg/kg with 23 participants), Cohort 6 (2.0 mg/kg with 23 participants), and Cohort 8 (2.88 mg/kg with 20 participants).
Dr. Sandra Aung, the Chief Clinical Officer at Ambrx, conveyed, “There have been no DLTs or SAEs recorded at the highest dose of ARX517 we’ve so far tested. We attribute this finding to the robustness of our conjugation approach, as supported by pharmacokinetic findings shared at the recent ESMO congress. Although there’s room to explore doses further, considerable and durable PSA declines have been noted at the 2.0 mg/kg level.”
Dr. Aung further mentioned, “The rapid enrollment and high level of interest in our protoporphyrin IX-based PSMA-targeted antibody-drug conjugate with the APEX-01 trial have been remarkable. Given the current trajectory, we are hopeful to establish a recommended dose for phase 2 trials by the beginning of the next year.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
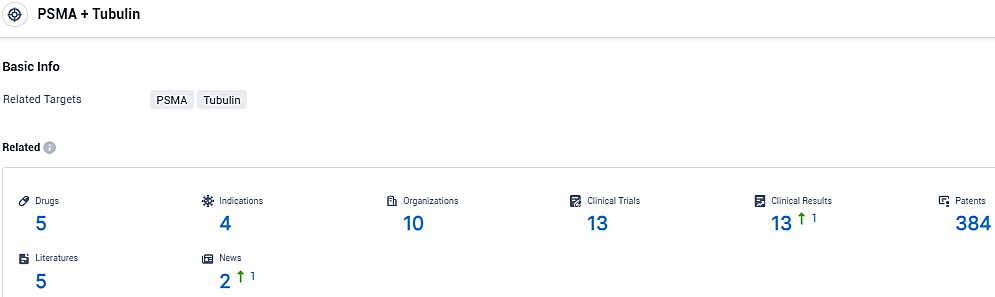
According to the data provided by the Synapse Database, As of December 7, 2023, there are 5 investigational drugs for the PSMA and Tubulin target, including 4 indications, 10 R&D institutions involved, with related clinical trials reaching 13, and as many as 384 patents.
ARX517 is an investigational antibody-drug conjugate composed of a humanized anti-PSMA mAb linked to AS269, an Ambrx proprietary potent microtubule inhibitor. ARX517 is designed to target the prostate-specific membrane antigen. PSMA is highly expressed in metastatic castration-resistant prostate cancer and has been validated as a therapeutic target.
ARX517 has the potential to be a first- and best-in-class anti-PSMA ADC addressing the high unmet medical need in mCRPC.