BioVie Reveals Results of Phase 3 Study for NE3107 Treating Early to Mid-Stage Alzheimer's Patients
BioVie Inc., a biopharmaceutical company engaged in the advancement of novel therapeutics for neurological and neurodegenerative conditions as well as severe liver ailments, has confirmed encouraging outcomes from a preliminary examination of key efficacy outcomes, which were not masked, from its pivotal Phase 3 study evaluating NE3107 for patients with mild to moderate Alzheimer’s Disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
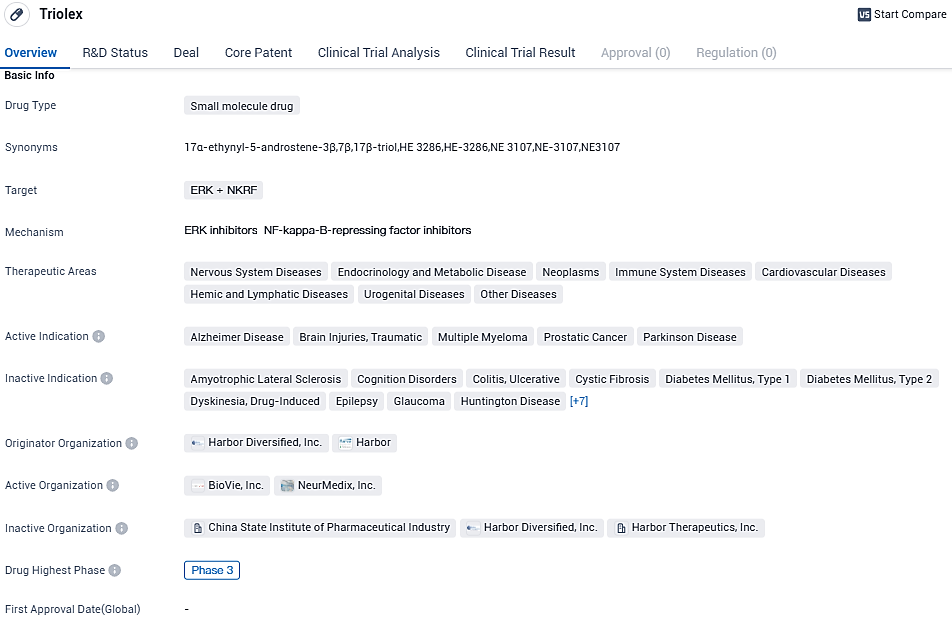
Clinical findings from patients deemed eligible indicate NE3107's therapeutic benefit may compare favorably or even surpass that of existing AD monoclonal antibody therapies. Those administered NE3107 witnessed a substantial delay in biological aging of 4.66 years relative to placebo, gauged by epigenetic/DNA methylation metrics using the Skin Blood Clock.
Initiated amidst the global health crisis of COVID-19, which posed barriers to clinical site access, the study registered 439 participants across 39 locations. At the conclusion of the study, significant departures from the established protocol and numerous instances of non-adherence to Good Clinical Practice standards were discovered at 15 sites.
Such an extraordinary pattern of potential misconduct prompted the organization to eliminate data from these locations and to seek intervention from the FDA's Office of Scientific Investigations. After discarding the compromised data, 81 patients remained in the Modified Intent to Treat cohort, with 57 in the Per-Protocol group, comprising individuals who fulfilled trial commitments and were confirmed to have ingested the investigative medication via pharmacokinetic evaluations.
"Our findings suggest that the therapeutic impact of NE3107 against placebo could be comparable or superior to results observed in clinical evaluations of authorized AD treatments, without the linked safety hazards,” stated BioVie's President and CEO, Cuong Do. "Our team's commitment to ethical conduct is also commendable, as demonstrated by our swift efforts to uncover and refer the questionable sites to the FDA for unbiased scrutiny."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
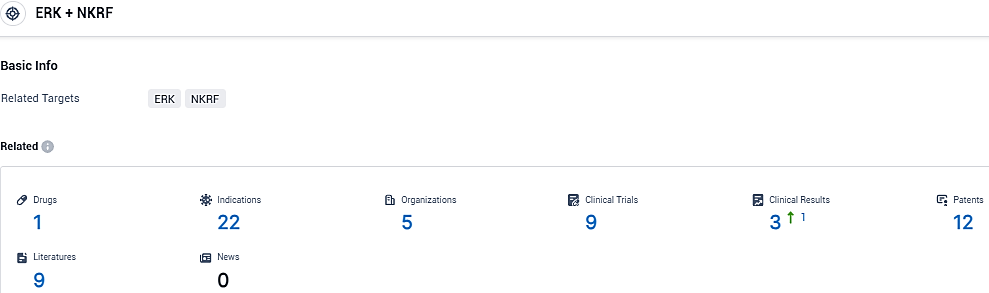
According to the data provided by the Synapse Database, As of December 7, 2023, there are 1 investigational drugs for the ERK and NKRF target, including 22 indications, 5 R&D institutions involved, with related clinical trials reaching 9, and as many as 12 patents.
NE3107 targets ERK and NKRF and is currently in Phase 3 of clinical development. With its broad therapeutic areas and active indications, Triolex has the potential to address various diseases, including Alzheimer's Disease, Brain Injuries, Multiple Myeloma, Prostatic Cancer, and Parkinson's Disease. The progress of Triolex to Phase 3 indicates promising results in earlier stages of testing, and if successful, it could potentially provide a valuable treatment option for patients in need.