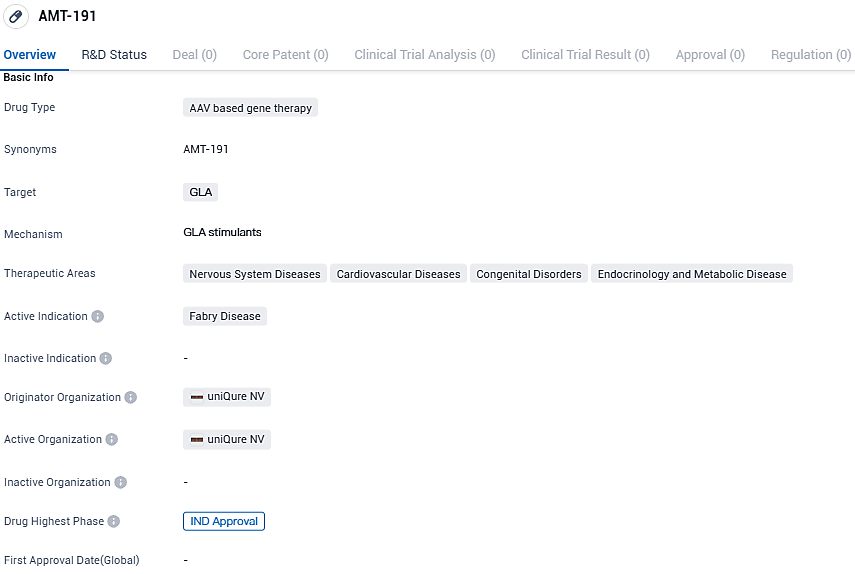
uniQure Reveals US FDA Approval for Clinical Trials of AMT-191, a Gene Therapy Candidate Targeting Fabry Disease
uniQure N.V., a foremost entity in the field of gene therapy, devoted to pioneering treatments that change the lives of individuals with critical health conditions, has disclosed that the FDA has granted authorization for the Investigational New Drug (IND) application for AMT-191. This particular gene therapy is being developed by the Company to combat Fabry disease. The therapeutic construct, known as AMT-191, utilizes an adeno-associated virus serotype 5 (AAV5) to transport an α-galactosidase A gene sequence specifically tailored to home in on the liver, with the intent to restore production of the deficient GLA protein.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The granting of the Investigational New Drug (IND) application for AMT-191 marks a pivotal point for the entity as it proclaims the advancement of a fourth therapeutic program into the clinical evaluation stage," announced Walid Abi-Saab, M.D., the principal medical officer at uniQure. "With the distinctive capability of serving as a one-off gene remediation method for Fabry disease, AMT-191 is a pioneering liver-specific gene therapy formulated for hemophilia B under development at uniQure. The architecture of our Phase I/II investigation aims to accumulate pivotal biomarker data that is both efficiently and economically obtained. We are enthusiastic about initiating the enlistment of our inaugural participant early in 2024."
The initial human clinical trial, categorized as Phase I/IIa, will transpire within the United States. This multi-site, unblinded study is structured with two ascending dose groups, each accommodating three individuals, to evaluate AMT-191's safety profile, tolerance, and potential therapeutic benefit in those affected by Fabry disease.
Representing an advanced AAV5-based gene therapy, AMT-191 consists of a GLA transgene, specifically engineered to home in on the liver and thereby induce the synthesis of the GLA protein. This therapy emerges as a significant innovation, offering a potentially one-time therapy for Fabry disease—a genetic disorder arising from a deleterious alteration in the GLA gene that results in a deficiency of the GLA enzyme. This enzyme shortfall leads to the progressive build-up of lipids within various cellular structures, culminating in a complex, multi-faceted pathological condition.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
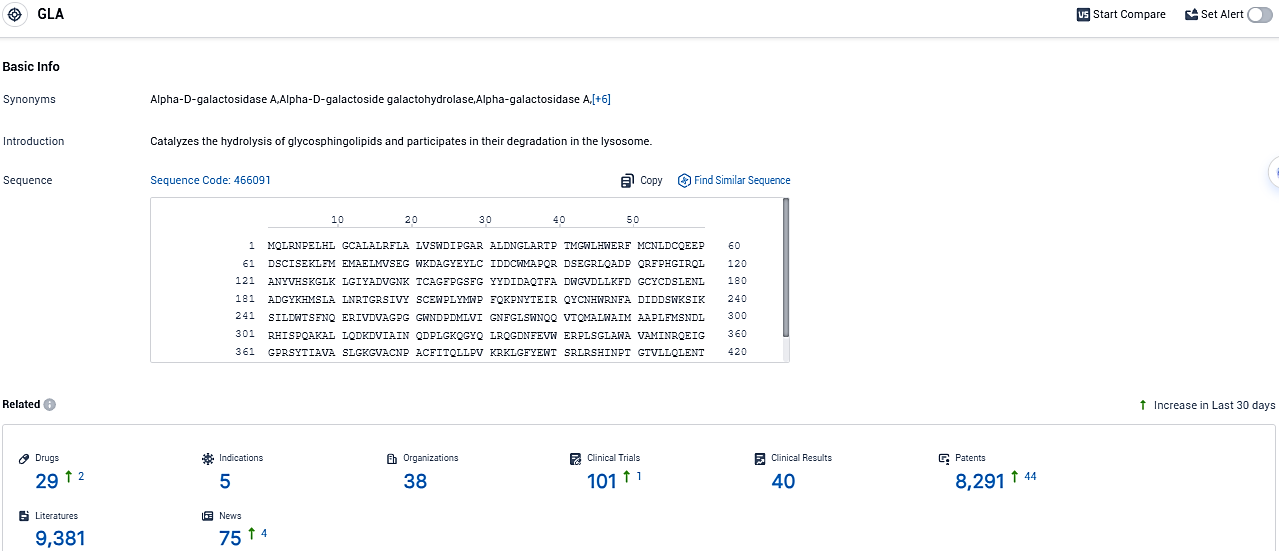
According to the data provided by the Synapse Database, As of December 7, 2023, there are 29 investigational drugs for the GLA target, including 5 indications, 38 R&D institutions involved, with related clinical trials reaching 101, and as many as 8291 patents.
The development of AMT-191 has reached the highest phase of IND approval. As an AAV-based gene therapy, AMT-191 represents an innovative and promising approach in the field of biomedicine. Further clinical trials and regulatory approvals will be necessary to determine its safety and efficacy in humans and to potentially bring this therapy to patients in need.