An analysis of RXC-004's R&D progress and its clinical results presented at the 2024 AACR Annual Meeting
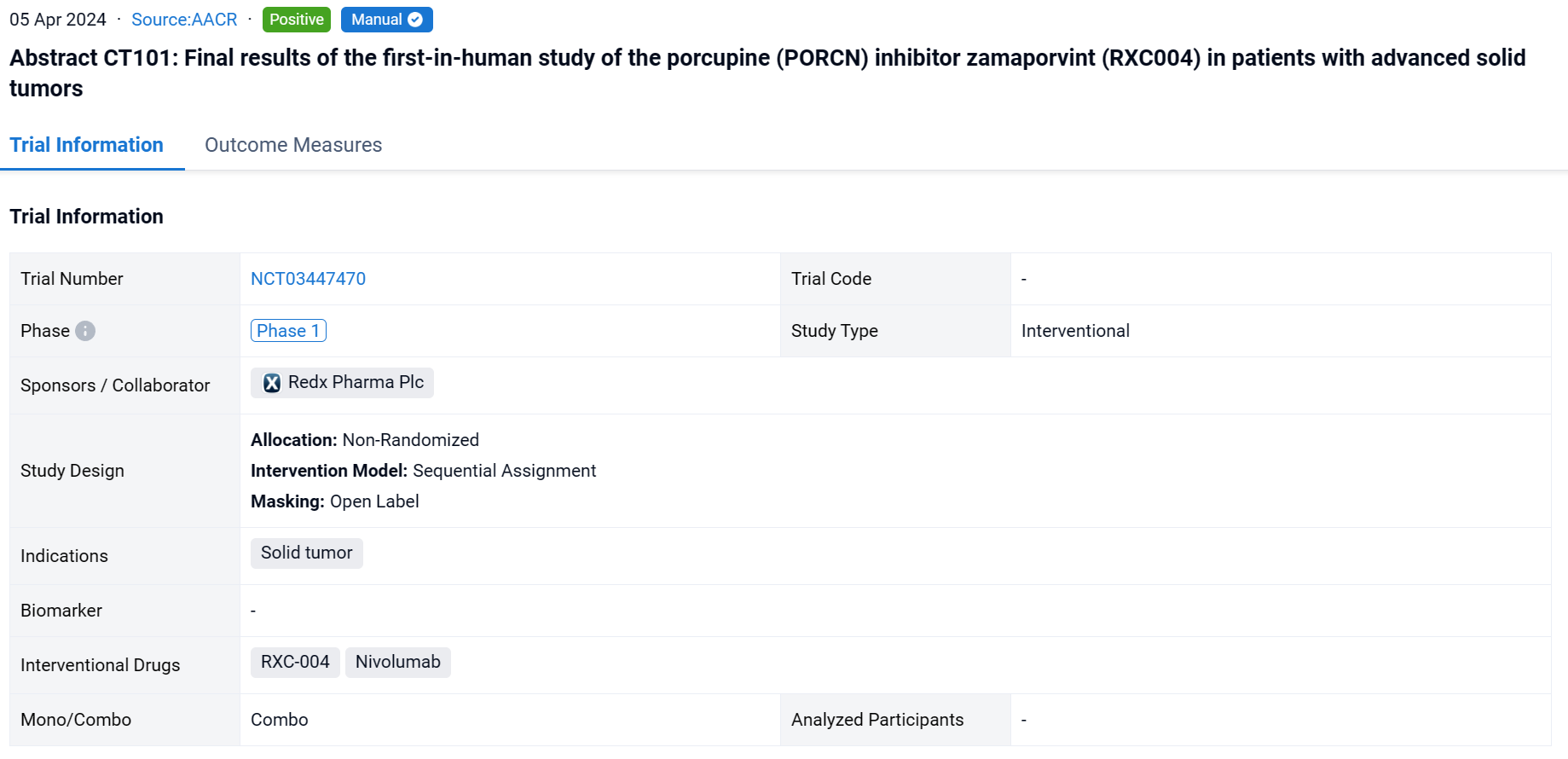
On April 5, 2024, the final results of the first-in-human study of the porcupine (PORCN) inhibitor zamaporvint (RXC004) in patients with advanced solid tumors were reported in 2024 AACR.
RXC-004's R&D Progress
RXC-004 is a small molecule drug that targets the protein PORCN. RXC-004 has shown potential in treating various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic diseases, and hemic and lymphatic diseases.
According to the Patsnap Synapse, RXC-004 is currently in Phase 2, which is the highest phase of clinical development globally. And the clinical trial distributions for RXC-004 are primarily in the United States, United Kingdom and Australia. The key indication is Metastatic Microsatellite Stable Colorectal Carcinoma.
Detailed Clinical Result of RXC-004
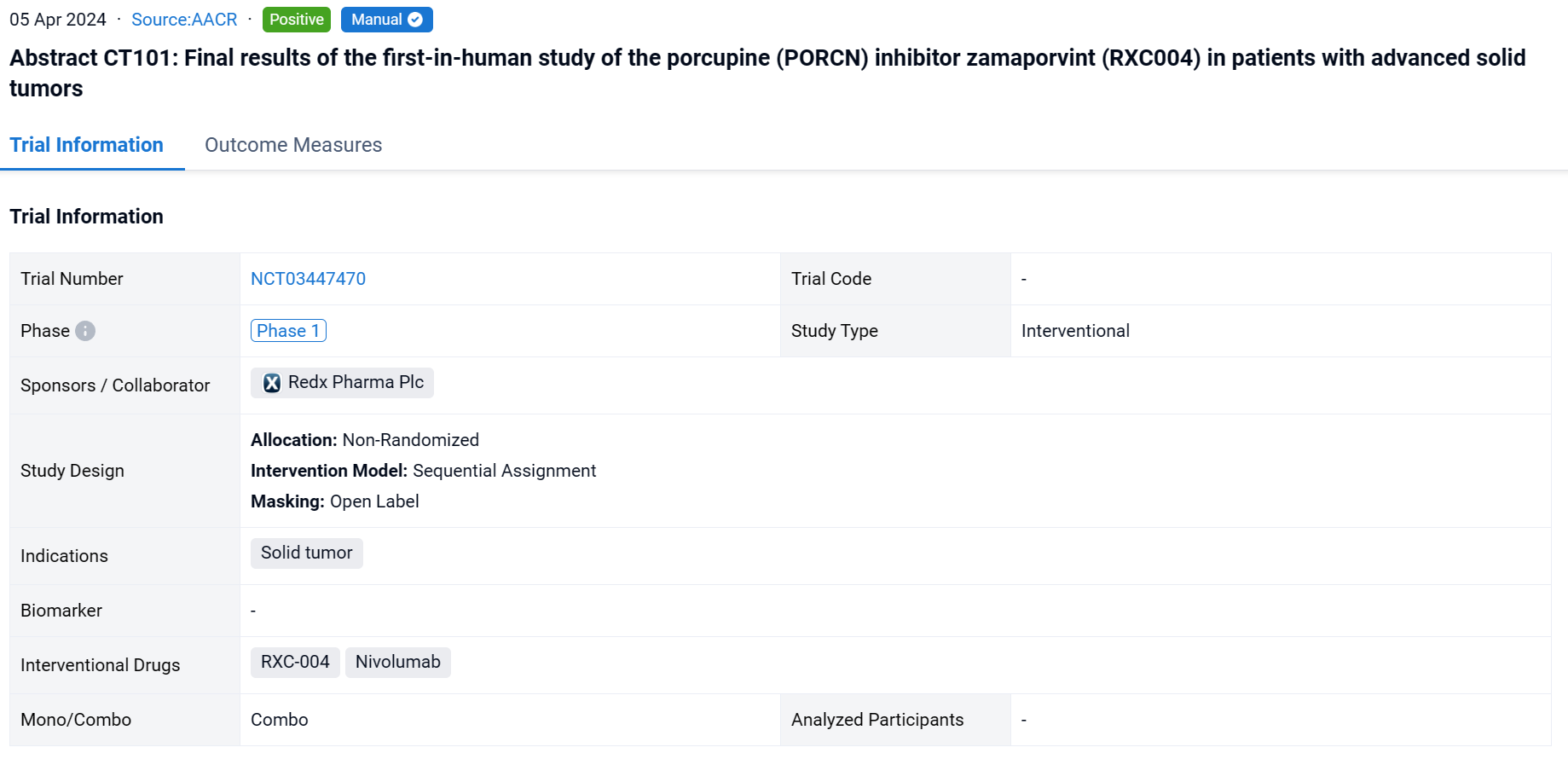
This non-randomized, sequential assignment, open-labeled clinical study (NCT03447470) was aimed to evacuate the efficacy and safety of RXC-004 in patients with advanced solid tumors.
In this study, module 1 investigated continuous monotherapy dosing, Module 2 investigated combination with nivolumab 480mg s.c. Q4W and Module 3 investigated intermittent monotherapy dosing (2 weeks on 1 week off). Patients received denosumab 120 mg s.c Q4W to prevent loss of bone mineral density (BMD), a known effect of Wnt inhibition. The primary objectives were safety and tolerability. Secondary objectives were PK and RECIST response. PD markers included on-treatment changes in: Wnt pathway and fibrosis biomarkers in paired skin biopsies; circulating immune subsets by flow cytometry; cytokine changes by Luminex, and changes in circulating-tumor DNA (ctDNA) levels by ddPCR.
The result showed that in module 1, 25 patients (pts) with unselected advanced solid tumors (AST) received RXC004 between 0.5mg and 10mg QD. The RP2D was 2mg QD; at this dose the most common AEs were fatigue, diarrhoea, dysgeusia and decreased appetite; no grade 4/5 AEs or bone fragility events were reported whilst 1 pt had a RXC004-related grade 3 AE, 3 pts (50%) had a dose interruption and 1 had a dose reduction. The median T1/2 of RXC004 was 14.5h. 5 pts with upstream Wnt pathway activated tumors had stable disease (SD), in 1 case for 26 weeks with sustained reduction in ctDNA and no BMD loss. In Module 2, 14 patients with unselected AST received RXC004 at 1mg QD or 1.5mg QD with nivolumab. The AE profile was similar to Module 1. The RP2D was 1.5mg QD; at this dose 3 pts had a RXC004-related grade 3 AE, 6 pts (75%) had a dose interruption and 3 had a dose reduction, and 4 pts had SD. PK was similar to Module 1. In Module 3, 7 patients with upstream Wnt-activated AST received RXC004 2mg QD, 2 weeks on, 1 week off. The AE profile was similar to Modules 1 and 2. There were no RXC004-related Grade 3 AEs, 2 pts (29%) had a dose interruption and there were no dose reductions. PK and PD analyses confirmed time off target. 2 pts had SD; 1 pt with RSPO fusion small intestinal adenocarcinoma remained on treatment for 36 weeks, with sustained reduction in ctDNA and no BMD loss.
It can be concluded that in patients with AST, RXC004 was safe and tolerated at doses up to 2mg QD as monotherapy and 1.5mg QD in combination with nivolumab, demonstrating differential disease control in upstream Wnt pathway activated tumours. Denosumab prevented loss of BMD. Intermittent RXC004 dosing was well tolerated with no detrimental impact on efficacy.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
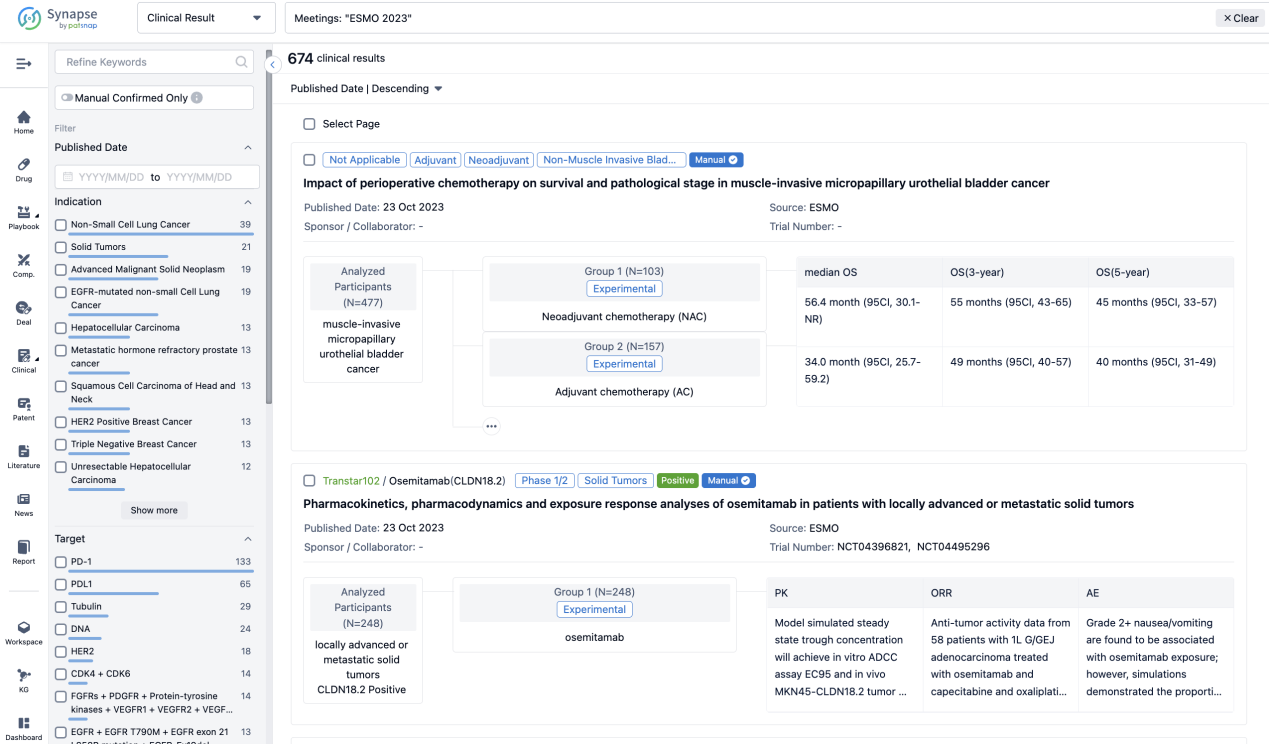
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.


Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!