Decoding Ociperlimab: a comprehensive study of its R&D trends and its clinical results in 2024 AACR
Despite a high response rate to cCRT, patients with limited-stage SCLC generally experience recurrence of disease after a few months and survival remains poor. Immunotherapy has shown benefit in many tumor types, including SCLC. In preclinical and clinical studies of solid tumors, co-inhibition of T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitor motif domains (TIGIT) and PD-1 enhanced antitumor activity of anti-PD-1. On April 7, 2024, the latest clinical data of ociperlimab + tislelizumab + cCRT in patients with untreated limited-stage SCLC was reported in 2024 AACR.
Ociperlimab's R&D Progress
Ociperlimab is a monoclonal antibody drug that targets TIGIT, a protein involved in immune regulation. Ociperlimab has shown potential in treating a wide range of therapeutic areas, including neoplasms (abnormal growth of cells), respiratory diseases, digestive system disorders, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
According to the Patsnap Synapse, Ociperlimab is currently in Phase 3 clinical trials, which is the highest phase of development both globally and in China. And the clinical trial distributions for Ociperlimab are primarily in the United States, China and France. The key indication is Non-Small Cell Lung Cancer.
Detailed Clinical Result of Ociperlimab
This phase 2, multicenter, randomized, 3-arm, open-label study investigating the preliminary efficacy and safety of ociperlimab (anti-TIGIT) + tislelizumab (anti-PD-1) + concurrent chemoradiotherapy (cCRT) in patients with untreated limited-stage small cell lung cancer (SCLC).
In this study, patients with limited-stage SCLC and no prior systemic therapy were randomized 1:1:1 to Arm A (ociperlimab [900 mg IV Q3W] + tislelizumab [200 mg IV Q3W] + cCRT for 4 cycles, then ociperlimab + tislelizumab), Arm B (tislelizumab + cCRT for 4 cycles, then tislelizumab), or Arm C (cCRT for 4 cycles). Study drugs (Arms A and B) were continued for up to 12 months or until progression, unacceptable toxicity, or withdrawal. Primary endpoint: investigator-assessed PFS per RECIST v1.1. Secondary analyses included additional efficacy and safety endpoints in the ITT population, and efficacy in patient subgroups by PD-L1 and TIGIT expression (both <1% vs ≥1%), using tumor area positivity (PD-L1) and immune cell scoring (TIGIT). No hypothesis testing was predefined (p-value for descriptive purposes only). Descriptive comparisons were conducted for Arm A vs C, B vs C, and A vs B.
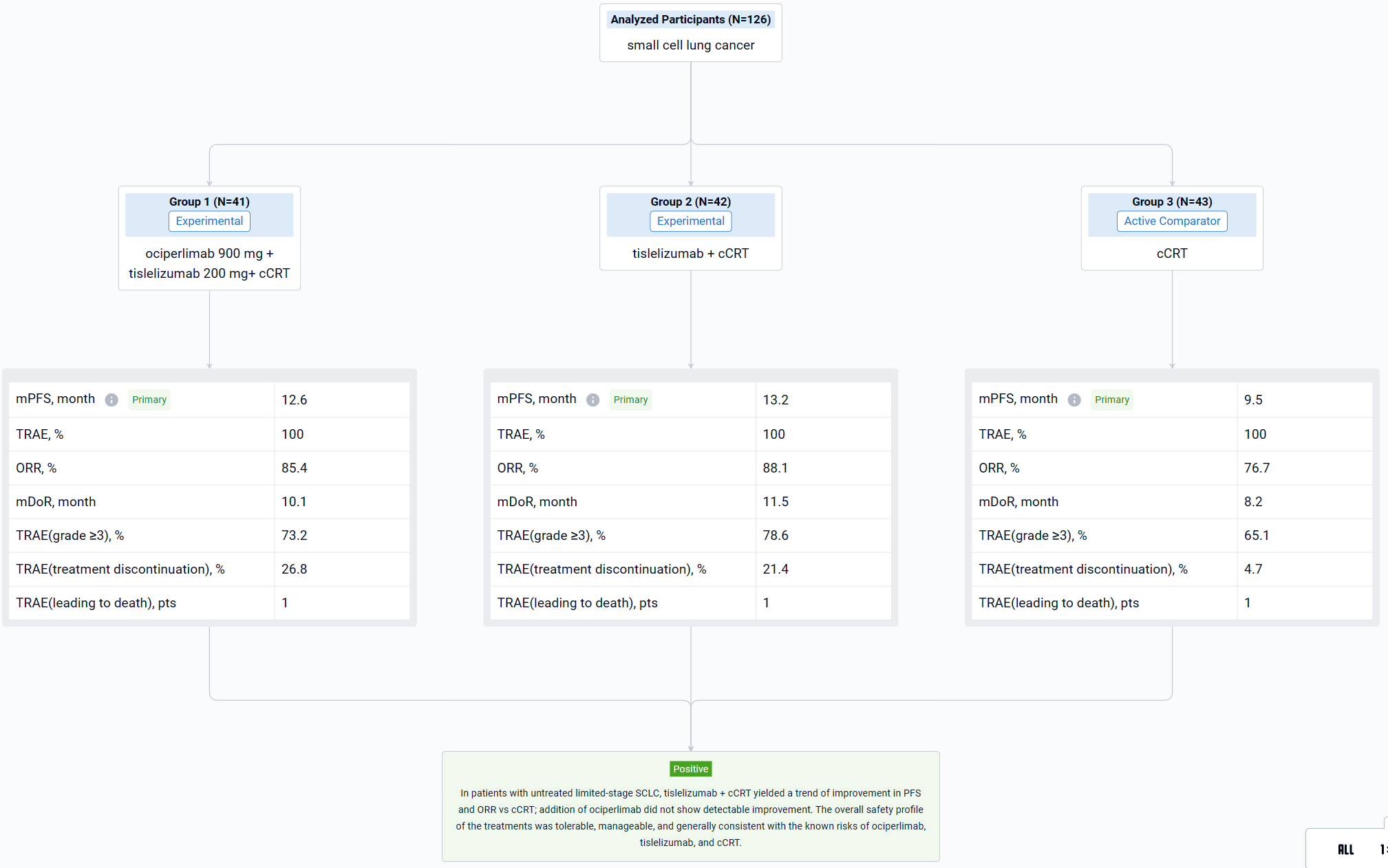
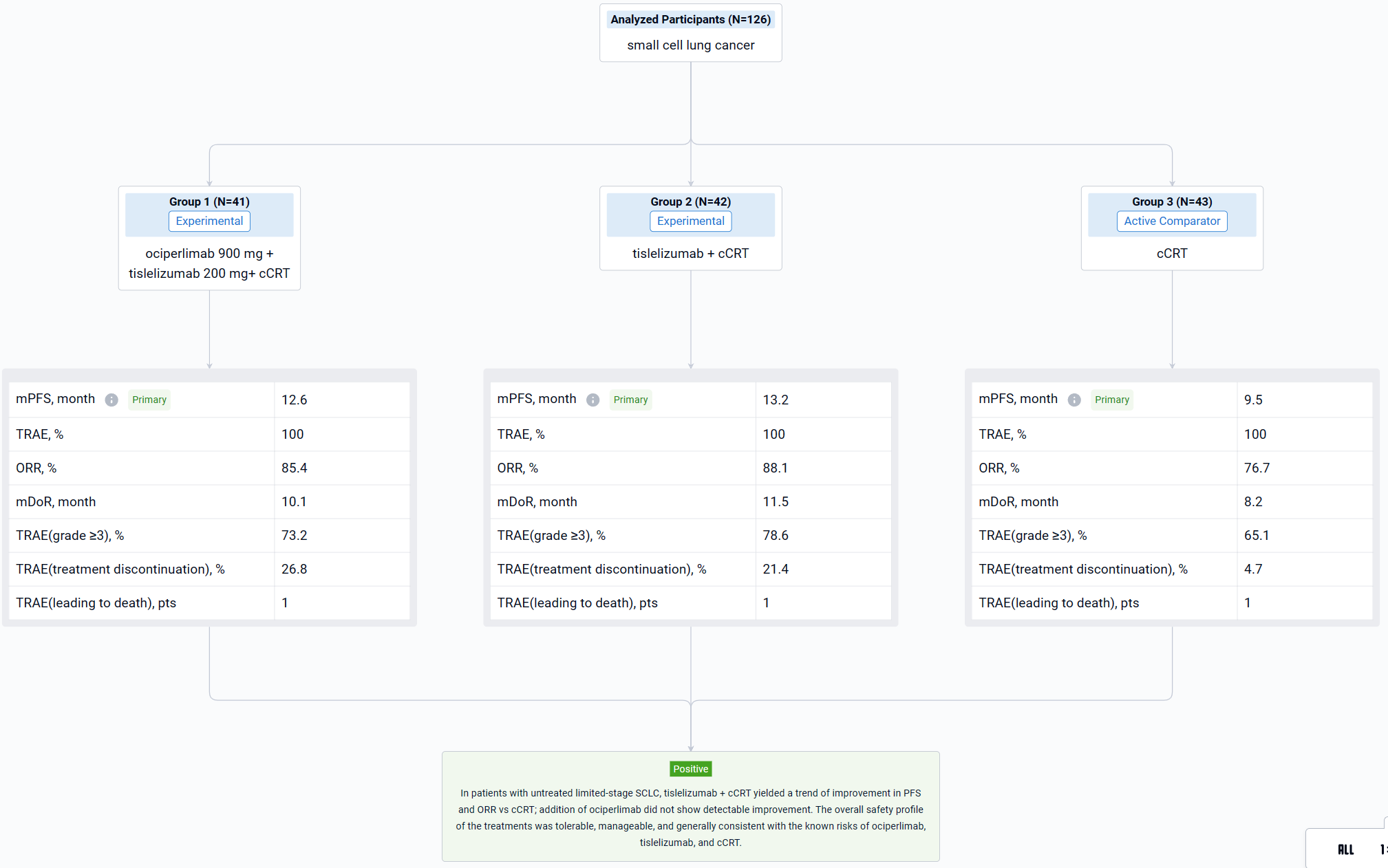
The result showed that as of July 26, 2023, 126 patients (median age, 61.5 years) were randomized to Arm A (n=41), Arm B (n=42), or Arm C (n=43). Median follow-up: ~18 months (all arms). There was a trend of improvement in median PFS in Arm A (12.6 months) and Arm B (13.2 months) vs Arm C (9.5 months); HR (95% CI): Arm A vs C, 0.84 (0.46-1.52; p=0.2793); Arm B vs C, 0.80 (0.45-1.44; p=0.2414). ORR was 85.4% (3 CR) in Arm A, 88.1% (4 CR) in Arm B, and 76.7% (1 CR) in Arm C. Median DoR was 10.1 months in Arm A, 11.5 months in Arm B, and 8.2 months in Arm C. Median OS was not reached in any arm. Analyses showed that PD-L1 or TIGIT expression did not correlate with efficacy, however, small subgroup size limits interpretability. All patients experienced ≥1 treatment-related adverse event (TRAE); rates of grade ≥3 TRAEs were 73.2%, 78.6% and, 65.1% in Arms A, B, and C, respectively. The most common TRAEs included anemia (80.5% in Arm A vs 83.3% in Arm B vs 81.4% in Arm C), nausea (80.5% vs 76.2% vs 65.1%), and WBC count decreased (78.0% vs 76.2% vs 62.8%). Rates of TRAEs leading to any treatment discontinuation were 26.8%, 21.4%, and 4.7% in Arms A, B, and C, respectively. One patient in each arm experienced a TRAE leading to death.
It can be concluded that in patients with untreated limited-stage SCLC, tislelizumab + cCRT yielded a trend of improvement in PFS and ORR vs cCRT; addition of ociperlimab did not show detectable improvement. The overall safety profile of the treatments was tolerable, manageable, and generally consistent with the known risks of ociperlimab, tislelizumab, and cCRT.
How to Easily View the Clinical Results Using Synapse Database?
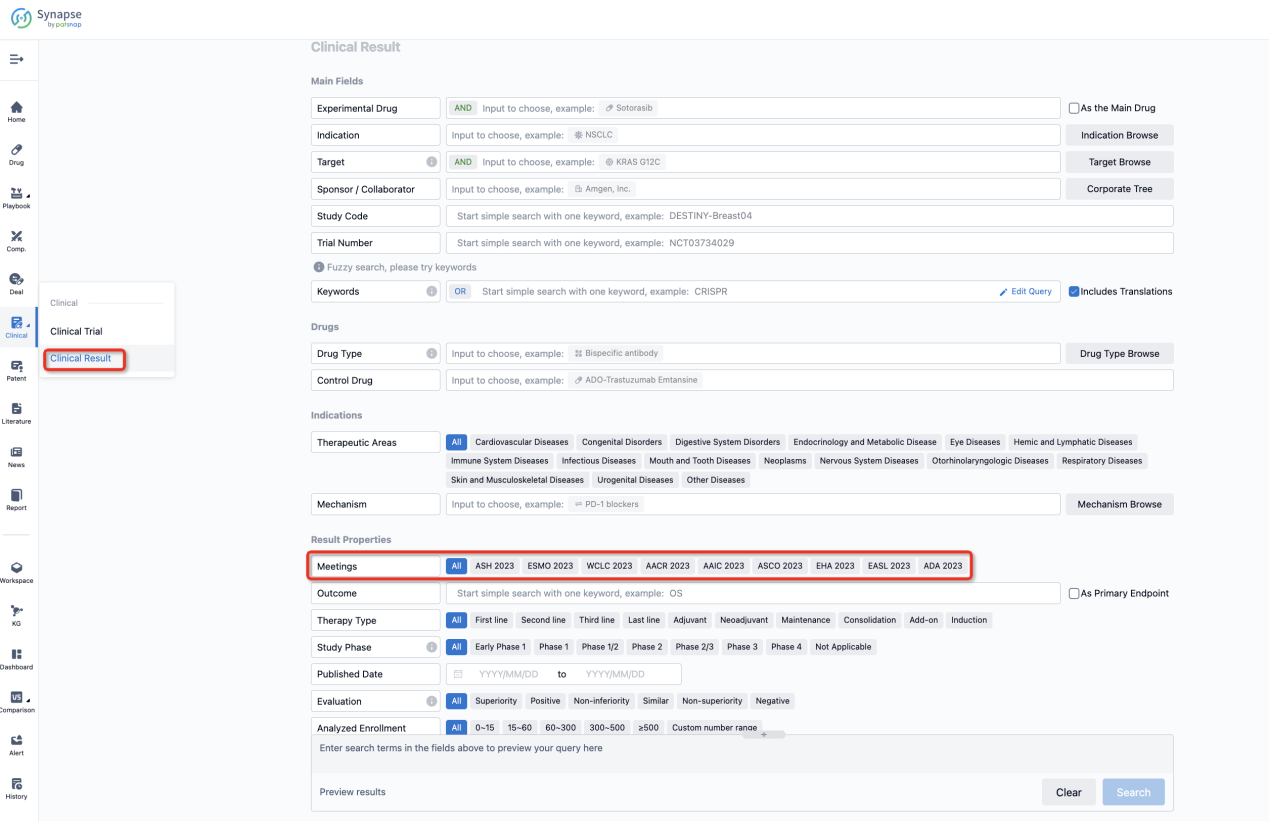
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
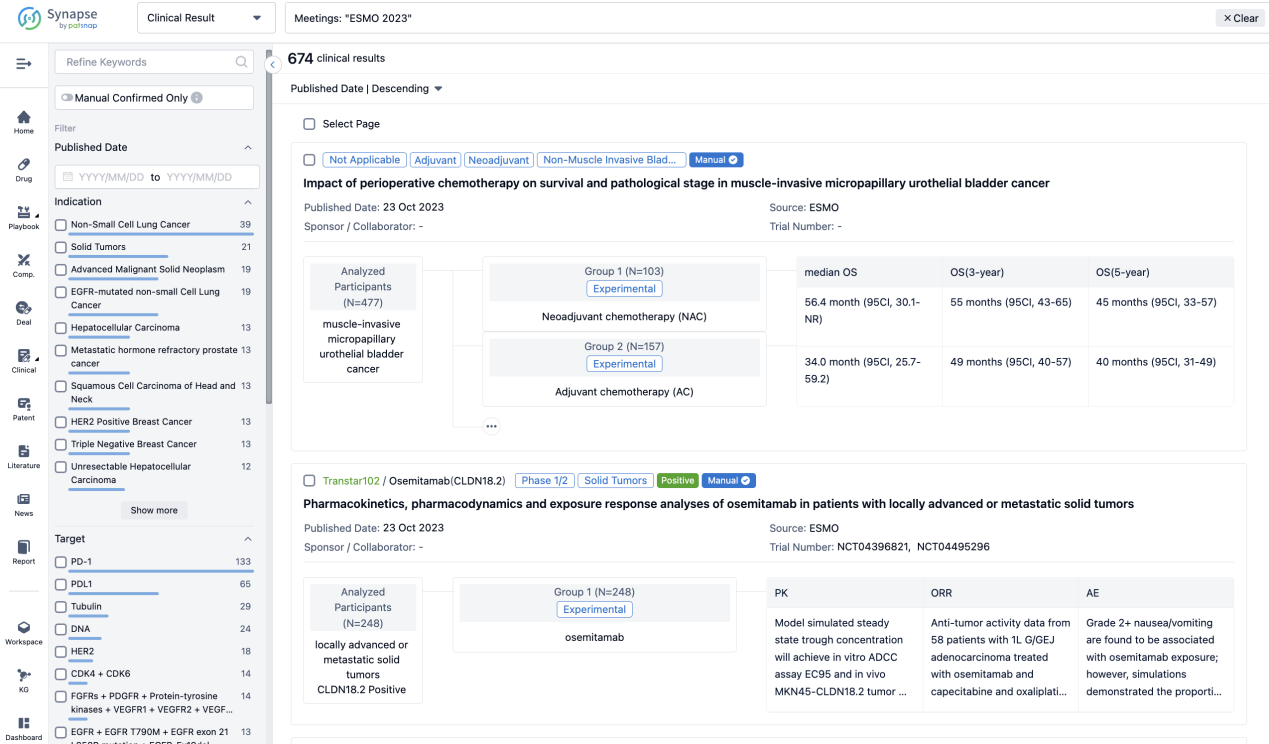
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
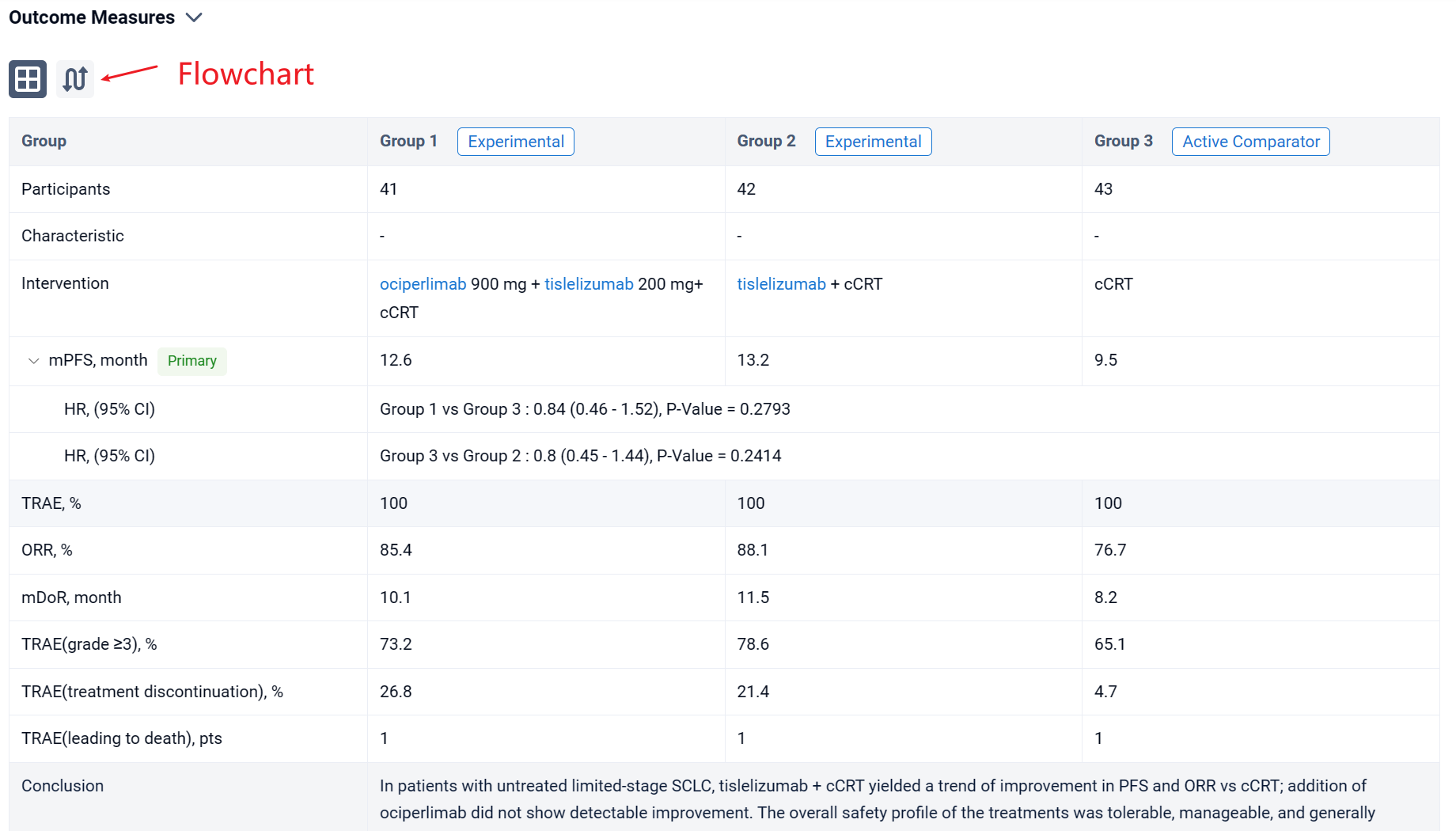
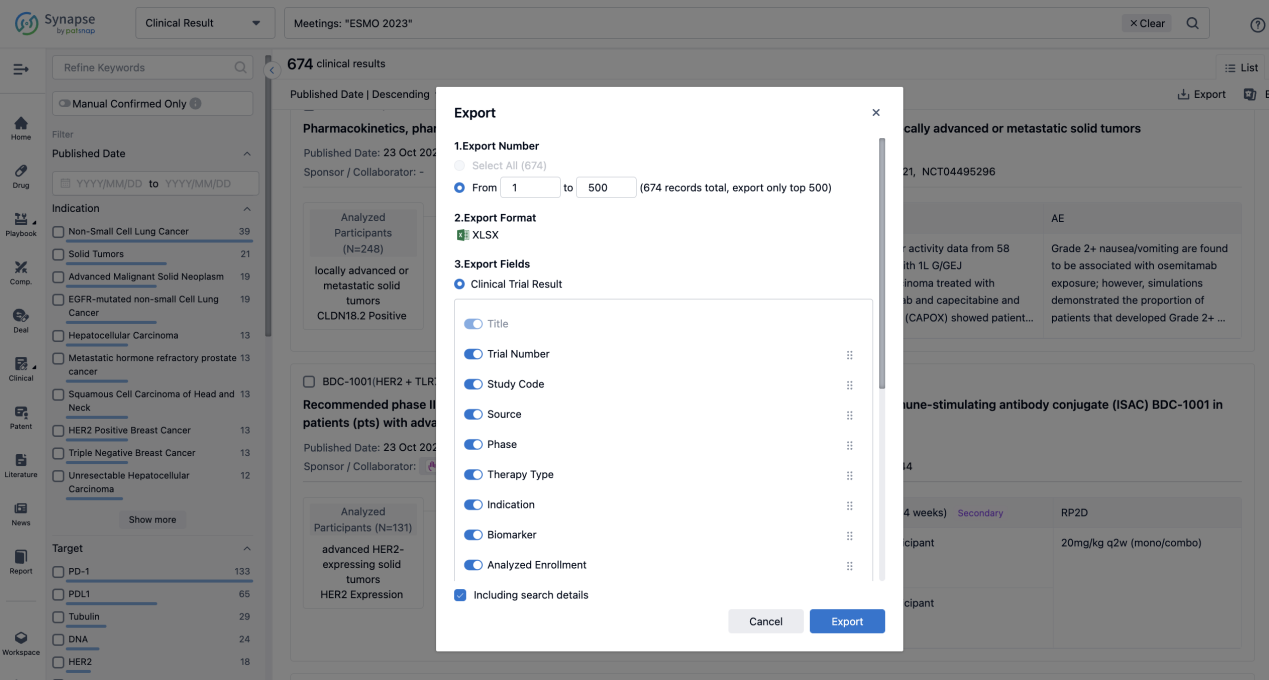
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!