Exploring the Latest Long Acting Injection Deal by Medincell: A Guide to Rapidly Accessing Transaction Insights
On April 16, 2024, Medincell announced that it had entered into a collaboration with AbbVie to co-develop and commercialize up to six long acting injection across multiple therapeutic areas and indications, such as Risperidone Extended-Release Injectable Suspension(Uzedy). Medincell will utilize its long-acting injectable technology platform to develop innovative therapies and conduct formulation activities and preclinical studies, including supporting CMC work, to advance drug candidates into clinical trials; AbbVie will provide funding and clinical development for each project and will be responsible for regulatory approval, manufacturing and commercialization.
Under the terms of the agreement, Medicell will receive an upfront payment of $35 million and is expected to receive up to $1.9 billion in development and commercial milestone ($315 million per program) fees, as well as single-digit to low-double-digit royalties on net sales.
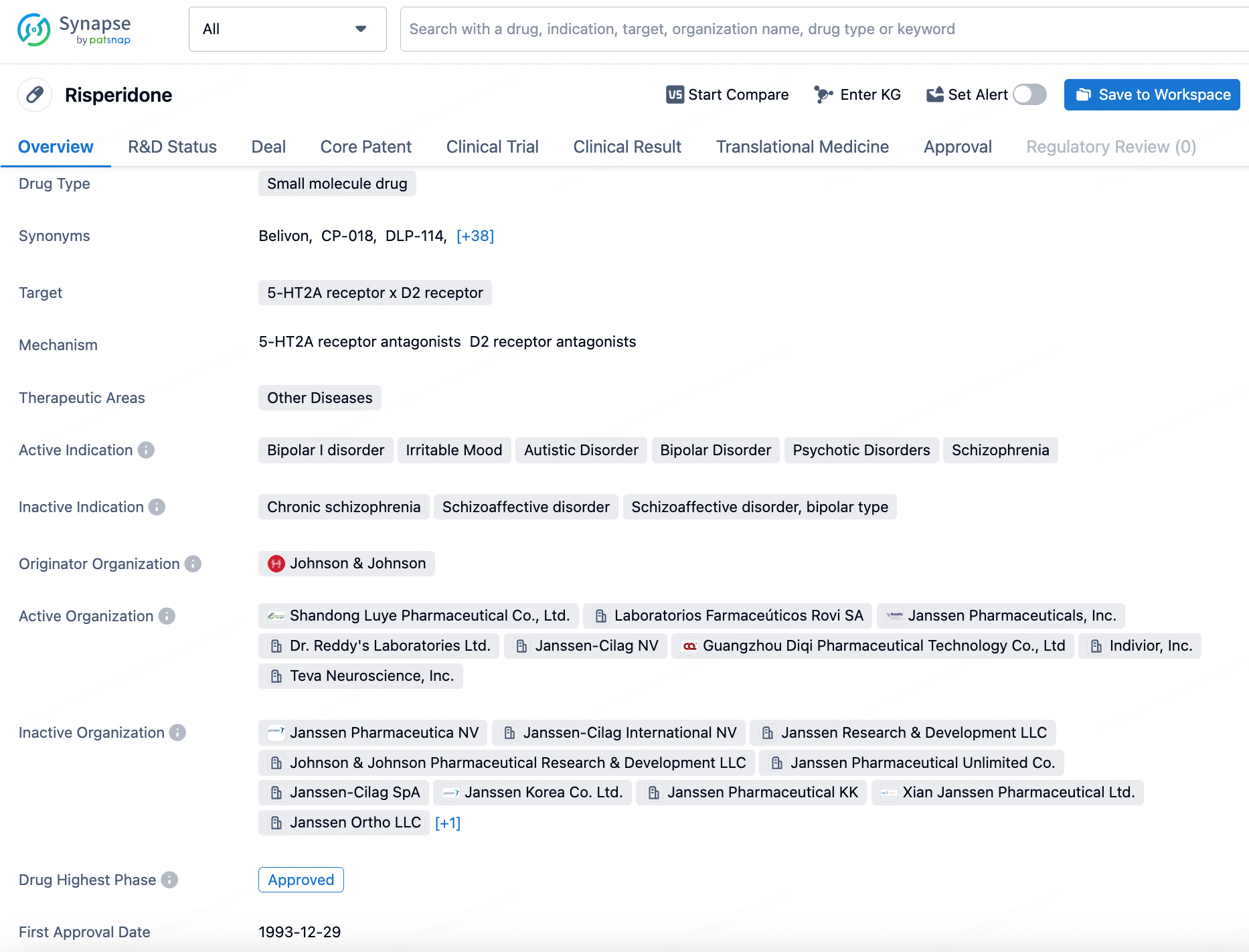
About Risperidone
Risperidone is a small molecule drug that primarily targets the 5-HT2A receptor and D2 receptor. It falls under the therapeutic area of Other Diseases, with active indications including Bipolar I disorder, Irritable Mood, Autistic Disorder, Bipolar Disorder, Psychotic Disorders, and Schizophrenia. Click the image below to directly embark on the exploration journey with the Risperidone!
MedinCell's only approved product, Uzedy (risperidone extended-release injectable suspension), developed in collaboration with Teva, was approved by the FDA in April 2023 for schizophrenia, which can be administered once every month or bimonthly. In the RISE trial, UZEDY prolonged time to relapse 5.0-fold with monthly dosing and 2.7-fold with bimonthly dosing. The risk of relapse was reduced by 80% and 62.5%, respectively, in patients with schizophrenia compared to placebo; additionally, the study results demonstrated that UZEDY provided clinically relevant plasma concentrations within 24 hours of dosing and maintained these concentrations at flexible dosing intervals. The safety profile was consistent with other approved risperidone formulations.
About MedinCell
MedinCell SA is a biopharmaceutical company based in France that was founded in 2002. It is a clinical and commercial stage biopharmaceutical company developing long-acting injectable drugs in many therapeutic areas.
According to MedinCell, its patented BEPO technology controls and ensures that drugs are administered regularly at optimal therapeutic doses over days, weeks or even months. Through subcutaneous or local injections, BEPO technology creates a polymer deposit of a few millimeters under the skin that exerts a systemic effect or a locally targeted effect. The deposits diffuse the active ingredient by absorption over the desired period of time, acting like a micropump that is both injectable and bioabsorbable.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
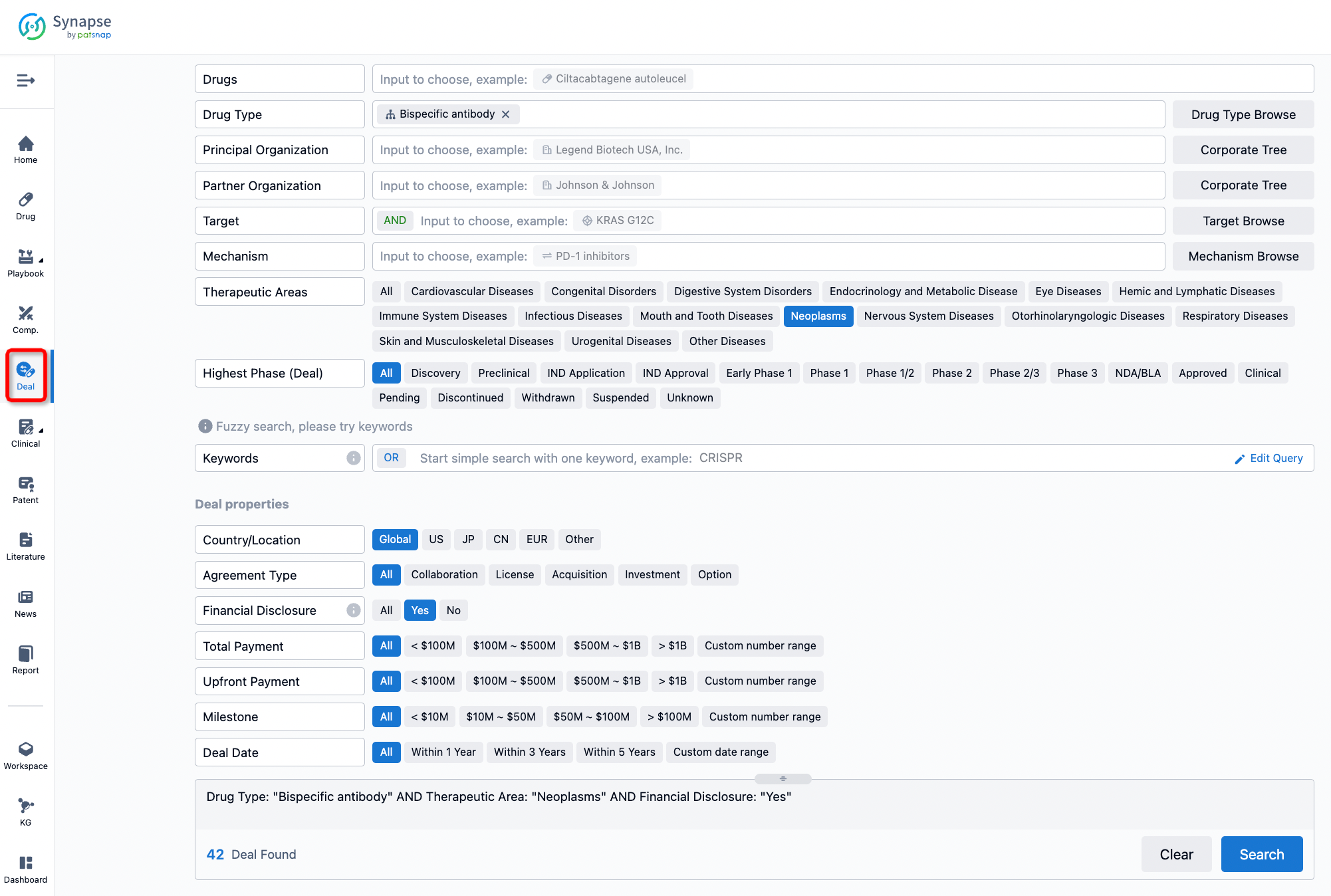
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
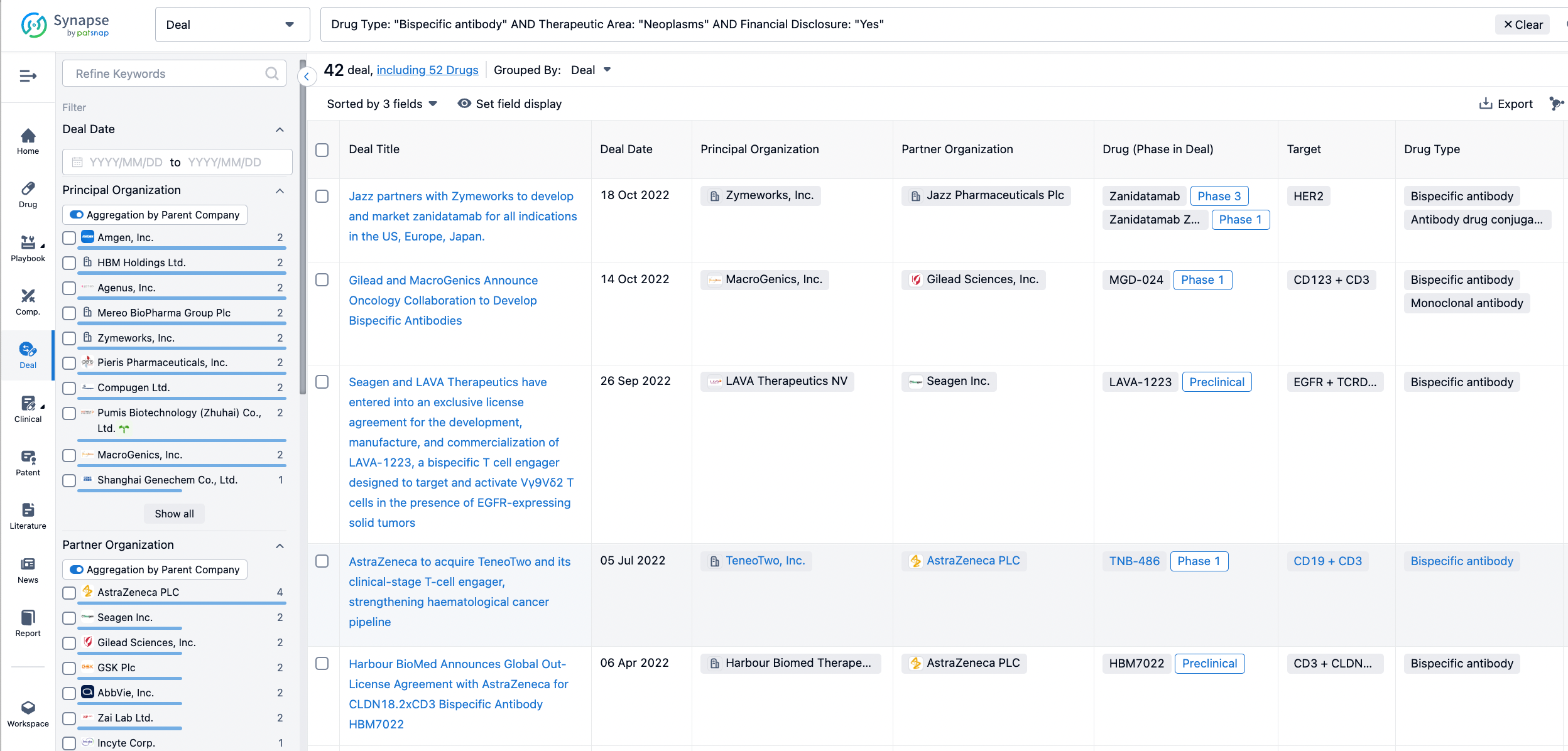
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
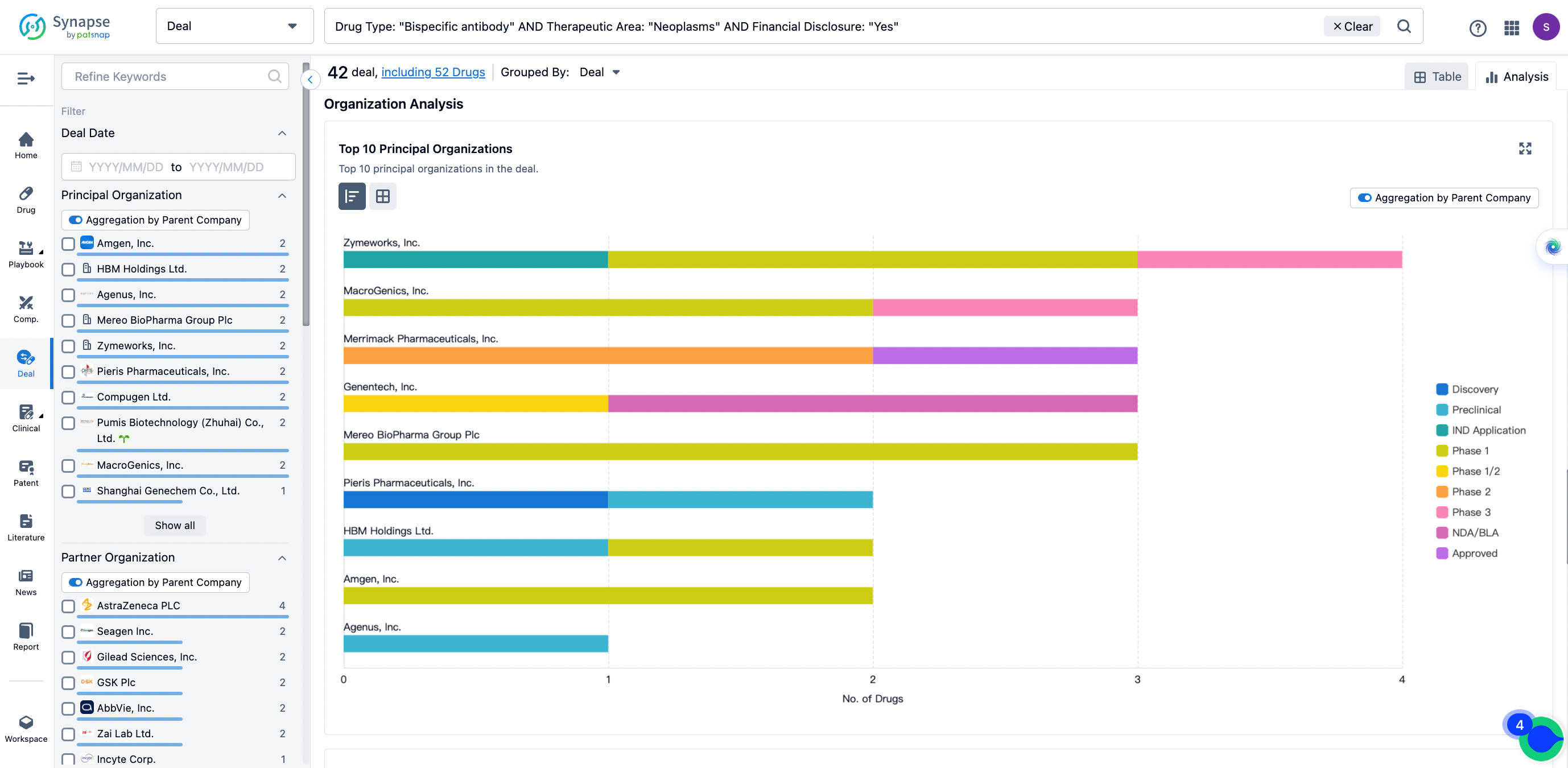
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
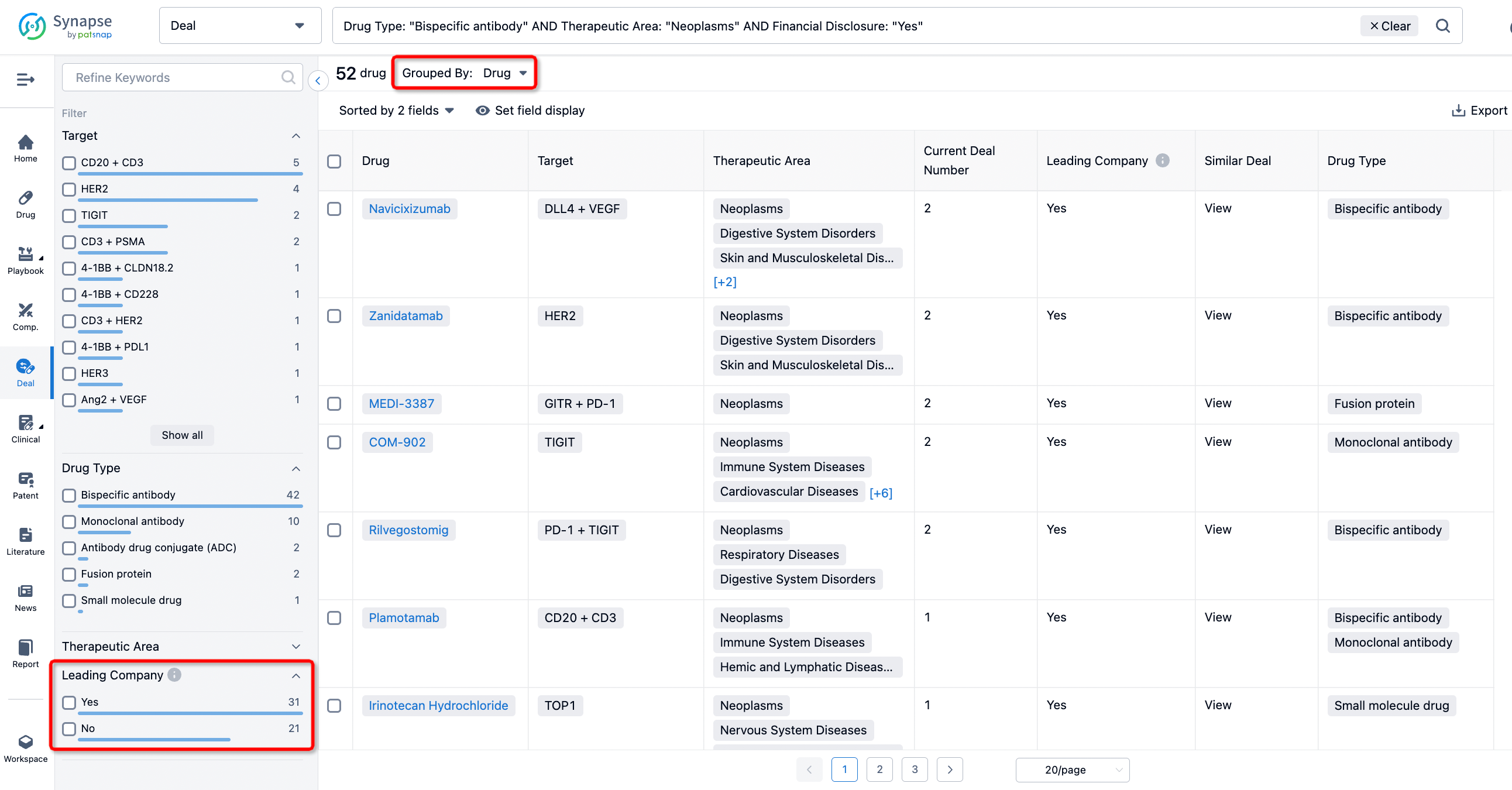
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.

Click on the image below to embark on a brand new journey of drug discovery!