An In-depth Analysis of Cephradine's R&D Progress and Mechanism of Action on Drug Target
Cephradine's R&D Progress
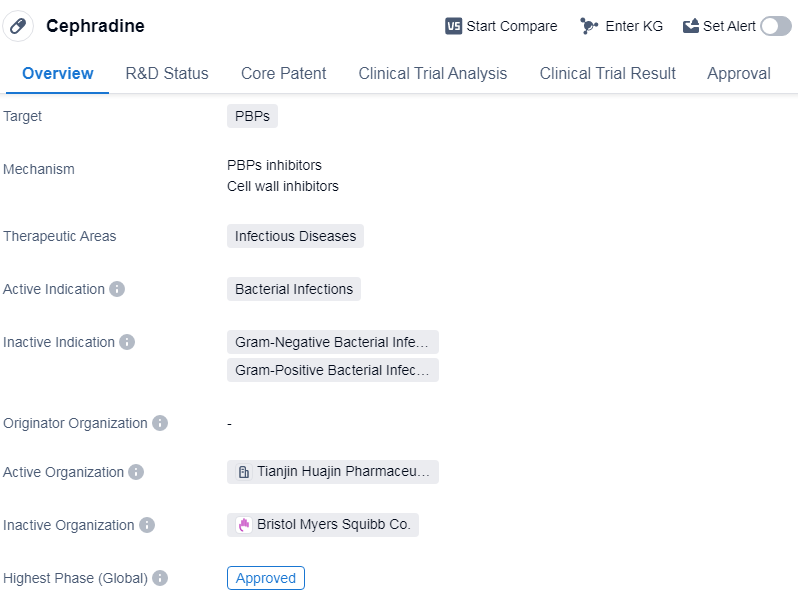
Cephradine is a small molecule drug that falls under the category of biomedicine. It primarily targets penicillin-binding proteins (PBPs) and is used in the treatment of infectious diseases caused by bacterial infections. The drug has been approved for use in global market.
Cephradine was first approved in China in January 1985, making it one of the early drugs to receive regulatory approval in the country. Since then, it has gained global recognition and has been approved for use in various countries worldwide. The drug's approval globally indicates its efficacy and safety profile in treating bacterial infections.
As a small molecule drug, Cephradine is designed to interact with specific penicillin-binding proteins, which are essential for bacterial cell wall synthesis. By targeting these proteins, the drug disrupts the bacterial cell wall formation, leading to the inhibition of bacterial growth and ultimately eliminating the infection.
The therapeutic areas in which Cephradine is primarily used are infectious diseases. Infectious diseases encompass a wide range of conditions caused by bacterial pathogens, and Cephradine's approval suggests its effectiveness in treating such infections.
With its being approved globally, Cephradine has undergone rigorous testing and evaluation to establish its safety and efficacy. The drug has been used for several decades, indicating its long-standing presence in the pharmaceutical market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cephradine: PBPs inhibitors and Cell wall inhibitors
From a biomedical perspective, PBPs inhibitors refer to a class of drugs that target PBPs. These proteins are essential for the synthesis of bacterial cell walls. By inhibiting PBPs, these drugs disrupt the formation of the bacterial cell wall, leading to cell lysis and ultimately killing the bacteria. This mechanism of action makes PBPs inhibitors effective against a wide range of bacterial infections.
Cell wall inhibitors, on the other hand, are a broader category of drugs that target various components involved in the synthesis or integrity of bacterial cell walls. These inhibitors can act on different stages of cell wall formation, such as blocking the synthesis of peptidoglycan or interfering with the cross-linking of cell wall components. By disrupting the cell wall structure, these drugs weaken the bacteria and make them more susceptible to the immune system or other antimicrobial agents.
In summary, PBPs inhibitors specifically target penicillin-binding proteins to disrupt bacterial cell wall synthesis, while cell wall inhibitors encompass a broader range of drugs that target different components involved in bacterial cell wall formation. Both types of inhibitors are important in the treatment of bacterial infections.
Drug Target R&D Trends for Cephradine
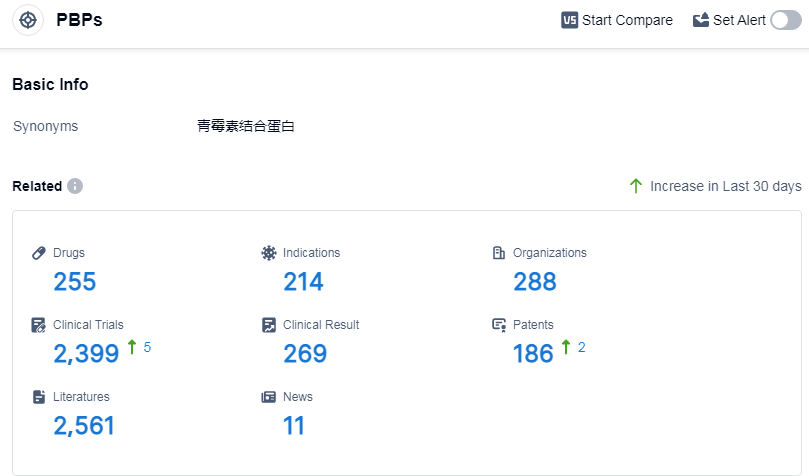
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 255 PBPs drugs worldwide, from 288 organizations, covering 214 indications, and conducting 2399 clinical trials.
The analysis of target PBPs in the pharmaceutical industry reveals that Pfizer Inc., Meiji Holdings Co., Ltd., Takeda Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Merck & Co., Inc. are the companies growing fastest under this target. The most common indications for drugs under target PBPs include bacterial infections, infectious diseases, and urinary tract infections. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly under this target, indicating potential competition with biosimilars. China, Japan, and the United States are the countries/locations developing fastest under this target, with China showing significant progress. The current competitive landscape suggests opportunities for collaborations and partnerships in the development of target PBPs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cephradine is a small molecule drug that targets penicillin-binding proteins and is primarily used in the treatment of infectious diseases caused by bacterial infections. It received its first approval in China in 1985 and has since gained global recognition. The drug's approval status and therapeutic areas highlight its effectiveness in combating bacterial infections, making it a valuable asset in the field of biomedicine.