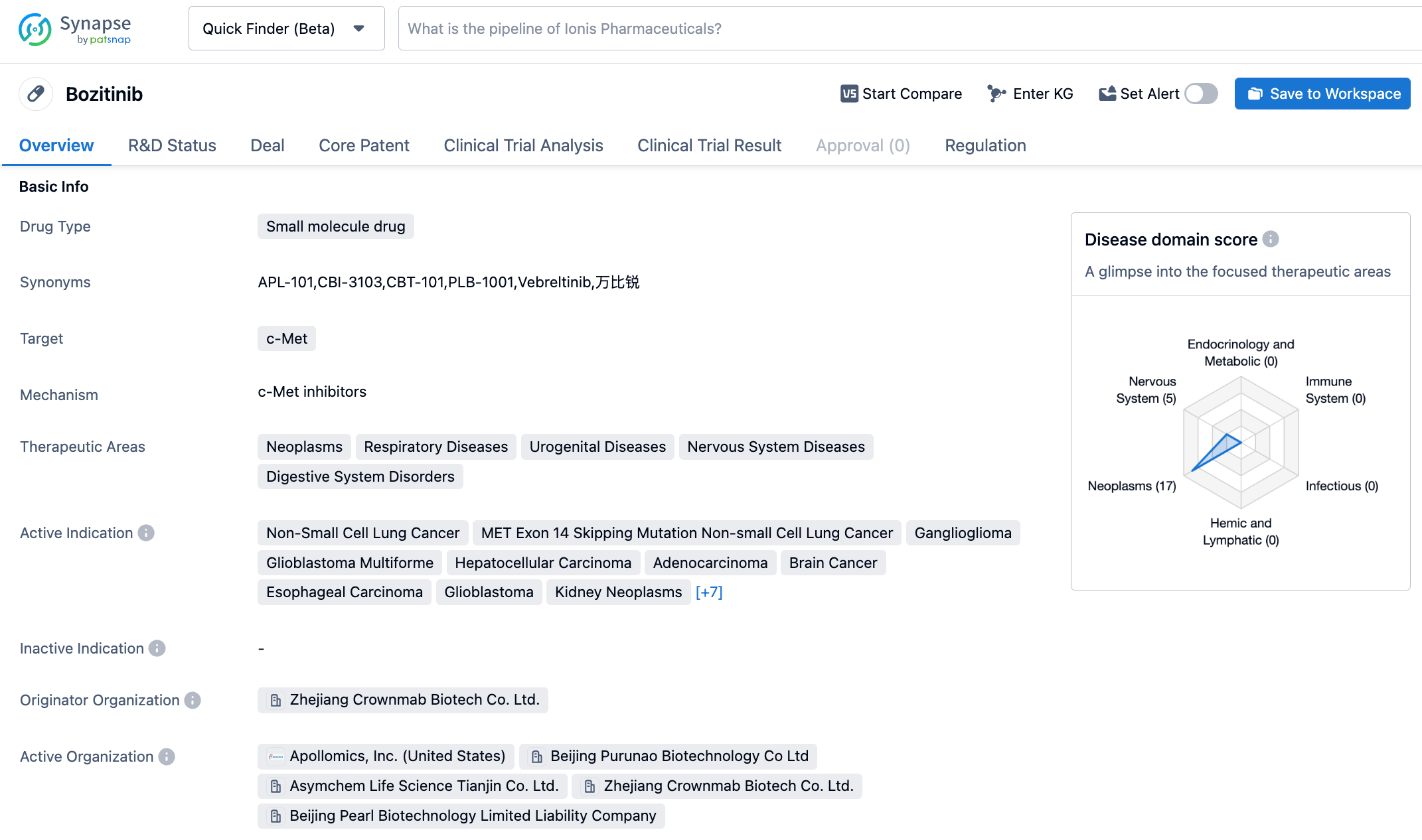
Anshi Biotechnology's small molecule selective MET inhibitor, Bozitinib, has applied for marketing for a new indication, the treatment of gliomatosis cerebri
On October 24, 2023, the Center for Drug Evaluation (CDE) of the National Medical Products Administration of China announced on its official website that Purunao Biotechnology, a wholly-owned subsidiary of Anshi Biotechnology, has applied for the listing of a new indication for the Class 1 new drug Vebreltinib (also known as beritinib) enteric-coated capsules. The proposed indication is for the treatment of adult patients with recurrent or intolerant IDH-mutant WHO grade IV astrocytoma or glioblastoma multiforme (GBM) with a history of lower-grade disease, carrying the PTPRZ1-MET fusion gene, after radiotherapy and temozolomide treatment.
Vebreltinib is a small molecule selective MET inhibitor, which has shown strong tumor inhibition in various preclinical models of MET-abnormal human gastric cancer, liver cancer, pancreatic cancer, and lung cancer cell xenografts (CDX) and patient-derived xenograft (PDX) mouse models. Currently, the drug has registered seven clinical trials in China, with indications involving NSCLC (Non-Small Cell Lung Cancer) and neuroglioma, etc. In February 2021, Vebreltinib was listed by CDE as a breakthrough treatment for NSCLC with MET Exon 14 Skipping mutation. On September 14, 2022, Vebreltinib was listed by CDE for priority review, and On September 24, 2022, “Vebreltinib Enteric-Coated Capsules”, a Class 1 new drug registration application, was officially accepted by the CDE.
At the 2023 ESMO meeting, Purunao Biotechnology, a wholly-owned subsidiary of Anshi Biotechnology, presented the Phase II trial results for Vebreltinib (Vebreltinib, bozitinib, PLB1001) in treating patients with MET Exon 14 Skipping mutations in the form of a poster. This comes after the company's disclosure of phase I study results for Vebreltinib at the 2020 AACR (American Association for Cancer Research) conference.
As of August 9, 2022, the study had enrolled 113 patients, 52 of whom had advanced Non-Small Cell Lung Cancer with METex14 Skipping mutations. 32.7% (17/52) of these patients had previously undergone systemic treatment for advanced or metastatic NSCLC. The primary endpoint, Blind Independent Review Committee (BIRC) assessed objective response rate (ORR), was 75% (95% CI: 61.1% ~ 86.0%), with an ORR of 77.1% (95% CI: 59.9% ~ 89.6%) in naive patients, and of 70.6% (95% CI: 44.0% ~ 89.7%) in treated patients. The Disease Control Rate (DCR) was 96.2%, the median Duration of Response (DoR) was 15.9 months, the median Time to Response (TTR) was 1.0 months, the median Progression-Free Survival (PFS) was 14.1 months, and the median Overall Survival (OS) was 20.7 months.
Patients with lung cancer brain metastases, liver metastases, and in the older age group (age ≥75 years) all benefited from Vebreltinib treatment, with ORRs of 100.0%, 66.7%, and 85.7%, respectively.
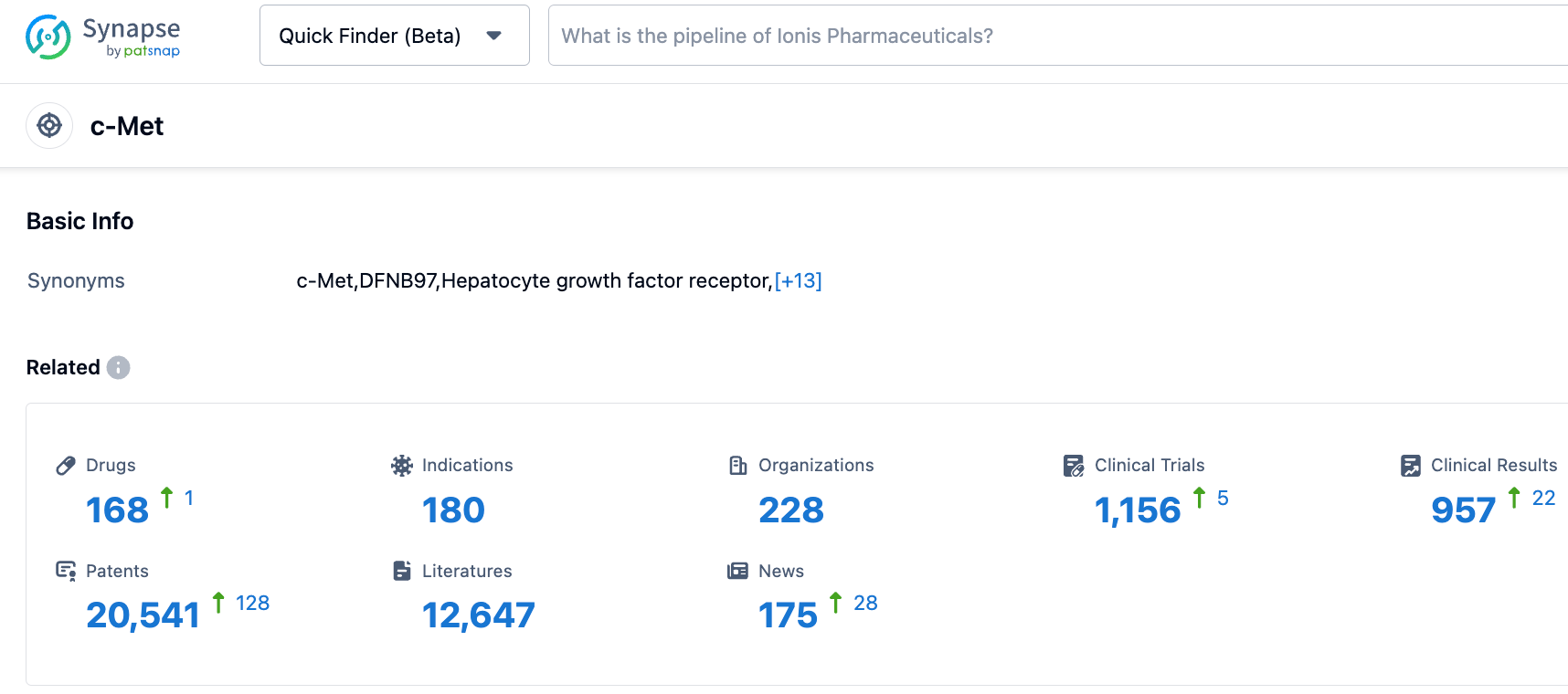
According to data disclosed by the Synapse database, as of October 26, 2023, there were 164 drugs under development for the c-MET target, covering 152 indications, 223 institutions with ongoing research, 1144 associated clinical trials, and as many as 20319 patents. For recurrent GBM patients who have failed standard treatment, especially those with positive ZM fusion gene GBM, there is currently no standard treatment. The hope is that the MET inhibitor Vebreltinib enteric-coated capsules will fill this therapeutic gap.