An In-depth Analysis of Lisocabtagene maraleucel's R&D Progress and Mechanism of Action on Drug Target
Lisocabtagene maraleucel's R&D Progress
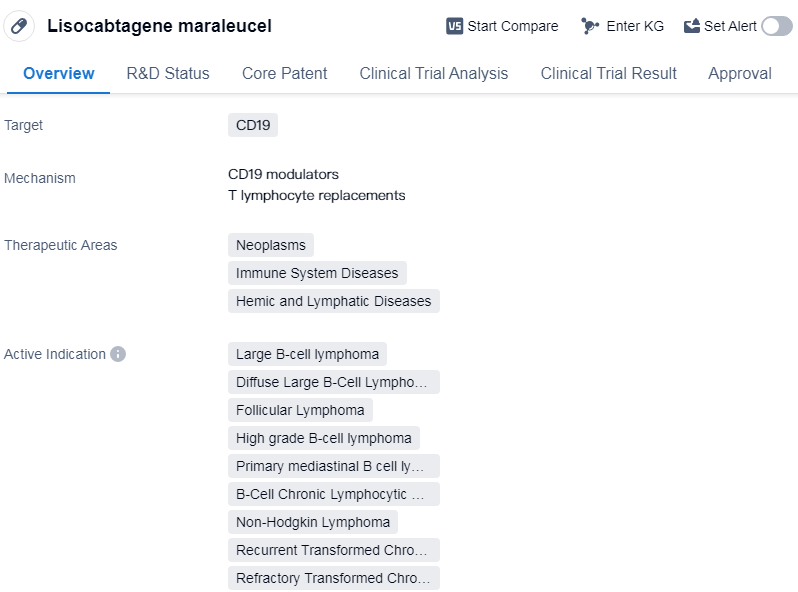
Lisocabtagene maraleucel is a CAR-T therapy that targets CD19, a protein found on the surface of B cells. It is primarily used in the treatment of various neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug has received its first approval in the United States in February 2021 and is indicated for the treatment of several types of lymphomas and leukemias.
The therapeutic areas in which lisocabtagene maraleucel is used include large B-cell lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, high-grade B-cell lymphoma, primary mediastinal B-cell lymphoma, etc.
The drug was developed by Bristol Myers Squibb Co. in collaboration with the Fred Hutchinson Cancer Research Center. It has reached the highest phase of development, which is approval, indicating that it has successfully completed clinical trials and demonstrated its safety and efficacy.
Lisocabtagene maraleucel received priority review status and orphan drug designation, which suggests that it addresses an unmet medical need for a rare disease or condition. Priority review is granted to drugs that have the potential to provide significant improvements in the treatment, diagnosis, or prevention of a serious condition.
CAR-T therapies, like lisocabtagene maraleucel, are a promising approach in the field of biomedicine. They involve modifying a patient's own immune cells to recognize and attack cancer cells expressing specific proteins, such as CD19. This personalized treatment approach has shown remarkable results in certain types of lymphomas and leukemias, offering new hope for patients who have not responded to other treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lisocabtagene maraleucel: CD19 modulators and T lymphocyte replacements
CD19 modulators are substances or drugs that target and interact with the CD19 protein. CD19 is a cell surface protein found on B cells, a type of white blood cell involved in the immune response. CD19 modulators can either enhance or inhibit the function of CD19, depending on their mechanism of action.
From a biomedical perspective, CD19 modulators are of particular interest in the field of immunotherapy. They can be used to manipulate the activity of B cells, which play a crucial role in immune system regulation and the production of antibodies. By targeting CD19, these modulators can potentially modulate the immune response, leading to therapeutic benefits in various diseases, including cancer and autoimmune disorders.
T lymphocyte replacements refer to the replacement or augmentation of T lymphocytes, also known as T cells, in the body. T cells are a type of white blood cell that are essential for the immune system's ability to recognize and eliminate infected or abnormal cells.
In certain medical conditions, such as immunodeficiency disorders or cancer, the number or function of T cells may be compromised. T lymphocyte replacements involve the administration of T cells, either from a donor or through genetic engineering techniques, to restore or enhance the immune response.
In summary, CD19 modulators are substances that interact with the CD19 protein on B cells, potentially modulating the immune response. T lymphocyte replacements involve the replacement or augmentation of T cells to restore or enhance immune function.
Drug Target R&D Trends for Lisocabtagene maraleucel
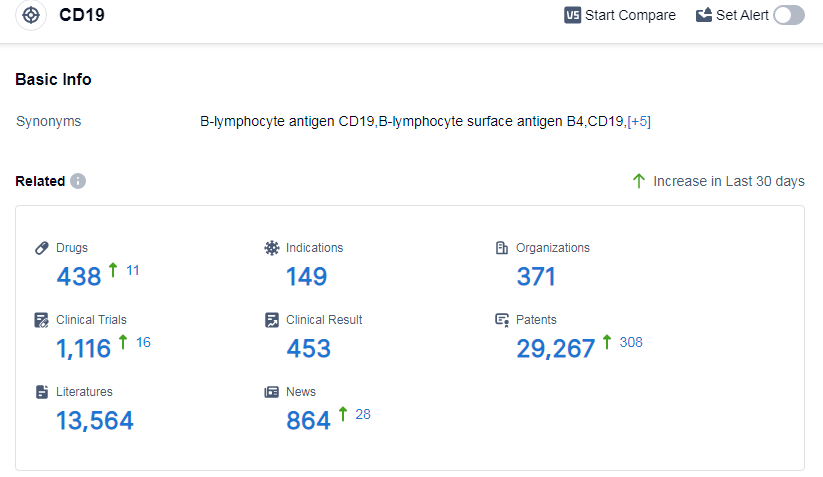
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 438 CD19 drugs worldwide, from 371 organizations, covering 149 indications, and conducting 1116 clinical trials.
The analysis of the current competitive landscape of target CD19 reveals that Gilead Sciences, Inc., Bristol Myers Squibb Co., Novartis AG, ADC Therapeutics SA, and JW Therapeutics (Shanghai) Co., Ltd. are the companies growing fastest in this field. These companies have made significant progress in the R&D of CD19-targeted therapies, with multiple drugs in advanced phases of development. The indications for which drugs have been approved include diffuse large B-cell lymphoma, follicular lymphoma, acute lymphoblastic leukemia, and other various lymphomas and leukemias.
CAR-T therapy is the most rapidly progressing drug type, followed by monoclonal antibody, bispecific antibody, and antibody drug conjugate (ADC). The United States, European Union, Japan, and China are the countries/locations developing fastest under the current targets, with China showing significant progress. Overall, the future development of target CD19 looks promising.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Lisocabtagene maraleucel is a CAR-T therapy that targets CD19 and has received approval for the treatment of various lymphomas and leukemias. Developed by Bristol Myers Squibb Co. and the Fred Hutchinson Cancer Research Center, it has shown promising results in clinical trials and has been granted priority review and orphan drug designation. This innovative therapy represents a significant advancement in the field of biomedicine and offers new treatment options for patients with certain types of blood cancers.