Molecular Glue: It bonds proteins and challenges the undruggable
Molecular glue refers to a class of small molecules that connect two or more proteins in a paste-like manner. Molecular glue can enhance and stabilize protein interactions; it can also repair protein interactions that have been weakened due to mutations. Furthermore, it induces the interaction of E3 ubiquitin ligase with protein targets, triggering target protein polyubiquitination and subsequent degradation by the proteasome. Molecular glue can also regulate protein functions; for example, PKM2 is a pyruvate kinase related to cancer. Its activity is low in homodimer form, but molecular glue can stabilize the dimer and "glue" two dimers together to form PKM2 homotetramer, thus increasing enzyme activity.
Targeted Protein Degradation (TPD) is a rapidly developing drug discovery strategy, using small molecules to recruit E3 ubiquitin ligase and promote protein hydrolysis. It has great potential in the treatment of tumors, infectious diseases, inflammation, and neurodegenerative diseases, and can break through the "undruggable" problem. With the rise of molecular glue degraders, these can induce or stabilize protein-protein interactions (PPI) between ubiquitin ligase and target proteins (substrates), leading to protein ubiquitination and subsequent proteasome degradation. Currently, several pharmaceutical companies are laying out in this field, mobilizing efforts to develop new therapies to meet unmet clinical needs.
Protein-Protein Interactions (PPIs) are vitally important for many cellular processes. Dysregulation of these interactions often leads to the onset of various diseases, such as cancer and Alzheimer's disease among others. Hence, when the dysregulation of protein interactions provokes disease, the development of drugs to regulate these interactions becomes a clinical target that needs to be addressed. Currently, the vast majority of drugs are protein inhibitors. However, many of the proteins causing disease are challenging to target with traditional small molecule drugs, these proteins are often referred to as "undruggable" targets.
Molecular glues can be utilized to develop drugs to target some of these "undruggable" entities. Molecular glues can enhance and stabilize Protein-Protein Interactions (PPIs), repair Protein-Protein Interactions (PPIs), and promote new Protein-Protein Interactions (PPIs). Molecular glues can regulate protein function by inhibiting or activating protein companions or by recruiting E3 ubiquitin ligase to degrade targeted protein complexes. Rationally designing molecular glues remains and will continue to be a challenge.
At present, most molecular glues and their mechanisms of action are typically discovered by chance. And screening for molecular glues involves high-throughput screening of small molecule compound libraries, a process akin to searching for a needle in a haystack. Looking forward, studying protein-protein interactions and the functional tertiary structure is crucial for designing molecular glues in a high-throughput fashion. We anticipate being able to more efficiently screen and even rationally design brand new molecular glues based on substrate structure in the future, for degrading a large number of "undruggable" disease targets in the body.
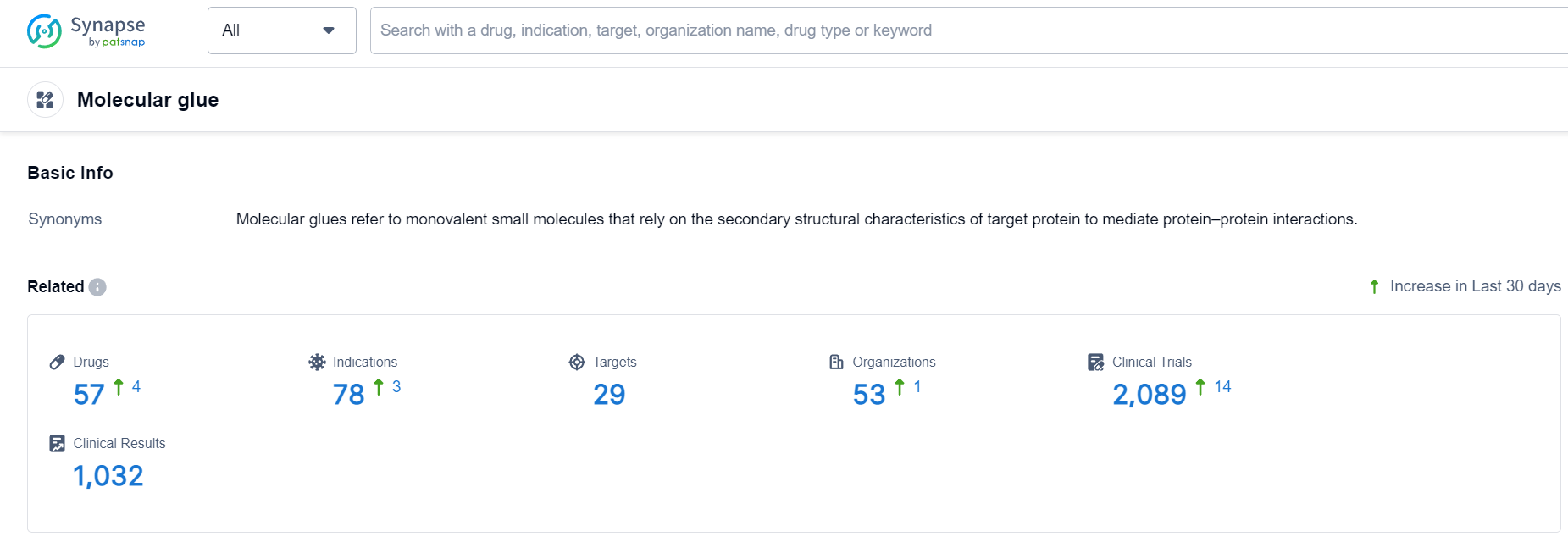
Molecular Glue Competitive Landscape
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 57 Molecular glue drugs worldwide, from 53 organizations, covering 29 targets, 78 indications, and conducting 2089 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
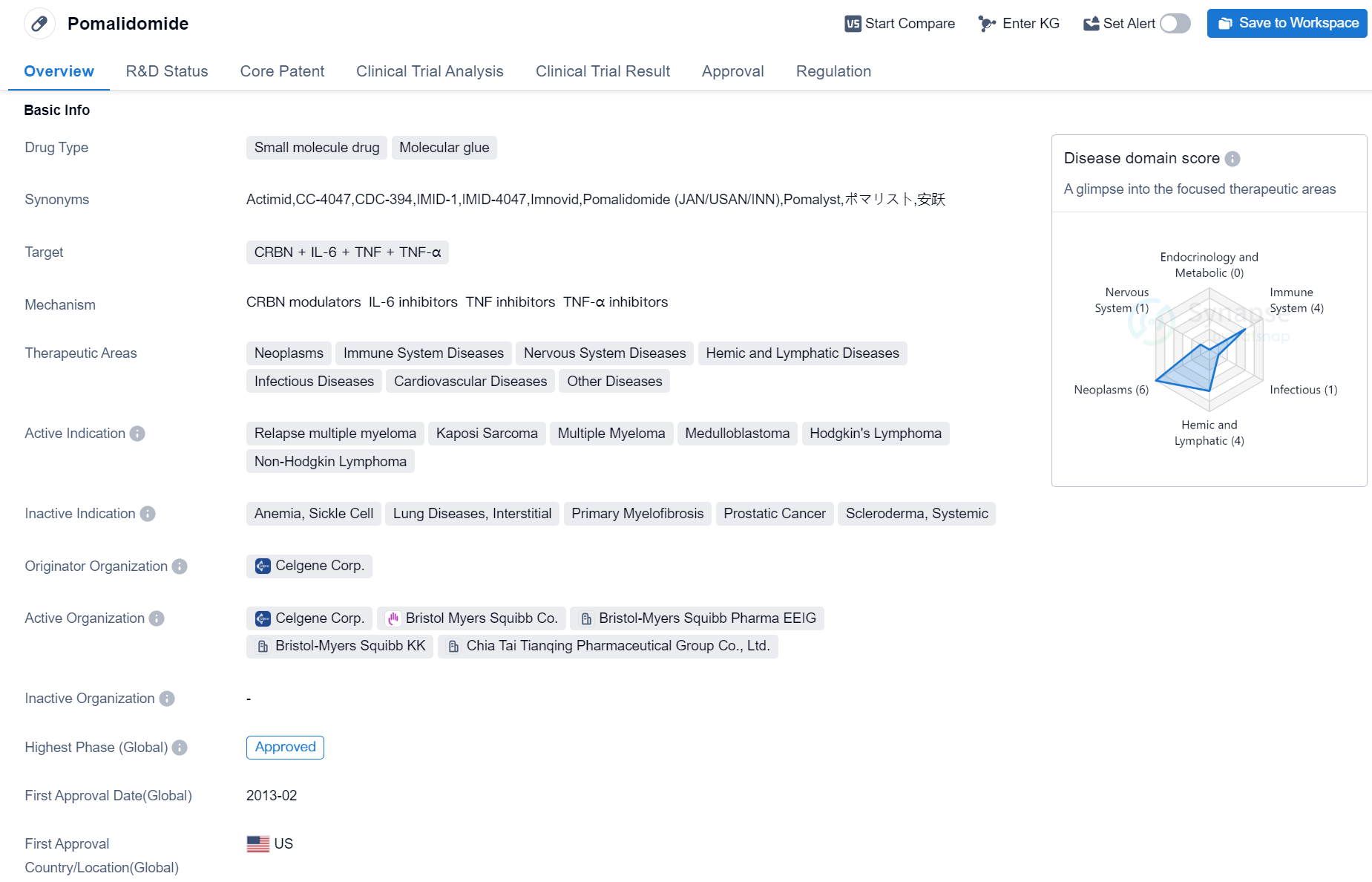
Approved Molecular Glue Medicinal: Pomalidomide
Pomalidomide is a small molecule drug classified as a molecular glue. It targets several proteins including CRBN, IL-6, TNF, and TNF-α. The drug has shown potential in treating various therapeutic areas such as neoplasms, immune system diseases, nervous system diseases, hemic and lymphatic diseases, infectious diseases, cardiovascular diseases, and other diseases.
Pomalidomide has been indicated for the treatment of relapse multiple myeloma, Kaposi Sarcoma, multiple myeloma, medulloblastoma, Hodgkin's Lymphoma, and non-Hodgkin lymphoma. It was developed by Celgene Corp., a pharmaceutical company known for its expertise in the field of biomedicine.
The drug has received approval for its highest phase in global market. Its first approval was granted in the United States in February 2013. Pomalidomide has undergone various regulatory processes, including priority review, accelerated approval, fast track, and orphan drug designation.
Pomalidomide's approval for multiple indications suggests its potential in addressing various diseases. Its small molecule nature and molecular glue classification indicate its ability to interact with specific proteins, potentially leading to therapeutic benefits. The drug's targeting of CRBN, IL-6, TNF, and TNF-α suggests its involvement in modulating immune responses and inflammatory processes.
Overall, Pomalidomide is a small molecule drug with a molecular glue classification that targets multiple proteins. Its approval for various therapeutic areas and indications suggests its potential in treating a wide range of diseases. The drug's regulatory designations and approval in multiple countries further support its potential as a valuable treatment option in the pharmaceutical industry.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
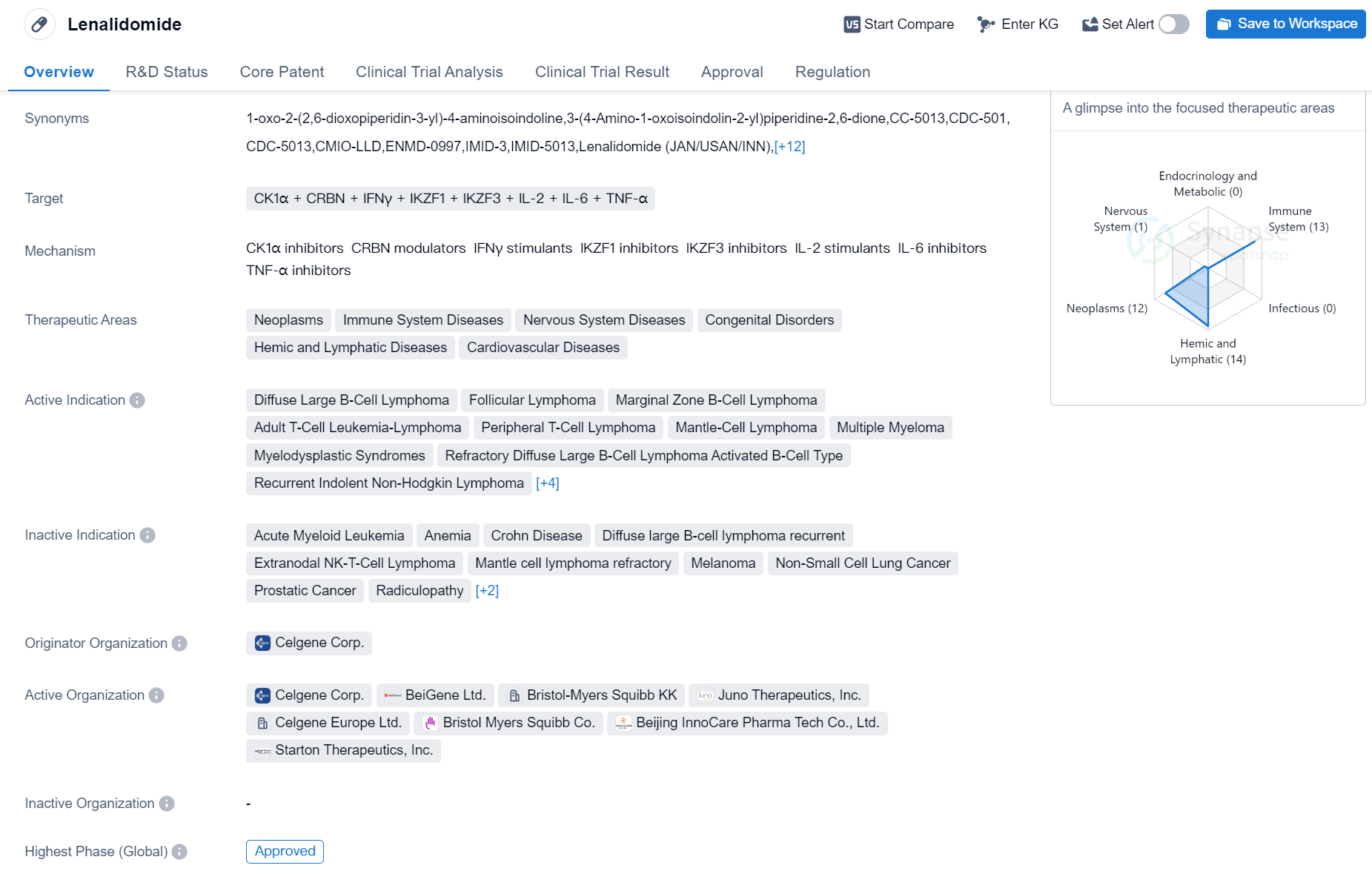
Lenalidomide
Lenalidomide is a small molecule drug classified as a molecular glue. It targets several proteins including CK1α, CRBN, IFNγ, IKZF1, IKZF3, IL-2, IL-6, and TNF-α. The drug has shown potential in treating various therapeutic areas such as neoplasms, immune system diseases, nervous system diseases, congenital disorders, hemic and lymphatic diseases, and cardiovascular diseases.
Lenalidomide has been approved for multiple indications globally, including Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Marginal Zone B-Cell Lymphoma, Adult T-Cell Leukemia-Lymphoma, Peripheral T-Cell Lymphoma, Mantle-Cell Lymphoma, Multiple Myeloma, Myelodysplastic Syndromes, Refractory Diffuse Large B-Cell Lymphoma Activated B-Cell Type, Recurrent Indolent Non-Hodgkin Lymphoma, Refractory Indolent Non-Hodgkin Lymphoma, B-Cell Chronic Lymphocytic Leukemia, B-Cell Lymphoma, and POEMS Syndrome.
The drug was first approved in the United States in December 2005 and has since gained approval in other countries. Lenalidomide is developed by Celgene Corp., which is the originator organization responsible for its discovery and development.
Lenalidomide has received regulatory designations such as Orphan Drug, Priority Review, and Special Review Project. These designations highlight the drug's potential to address unmet medical needs and expedite the regulatory review process.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, Lenalidomide is a small molecule drug developed by Celgene Corp. It targets several proteins and has been approved for various therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, congenital disorders, hemic and lymphatic diseases, and cardiovascular diseases. The drug has received regulatory designations and was first approved in the United States in 2005. Its ability to act as a molecular glue and target multiple proteins makes it a promising option for the treatment of several diseases.