The positive data from Apellis' Phase 3 study on pegcetacoplan was published in The Lancet, targeting the main cause of blindness in the elderly
Recently, Apellis Pharmaceuticals announced that The Lancet published positive 24-month results from the Phase 3 OAKS and DERBY studies evaluating Syfovre (pegcetacoplan) in the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The results showed significant reduction in GA lesion growth with both every other month and once monthly treatment with Syfovre.
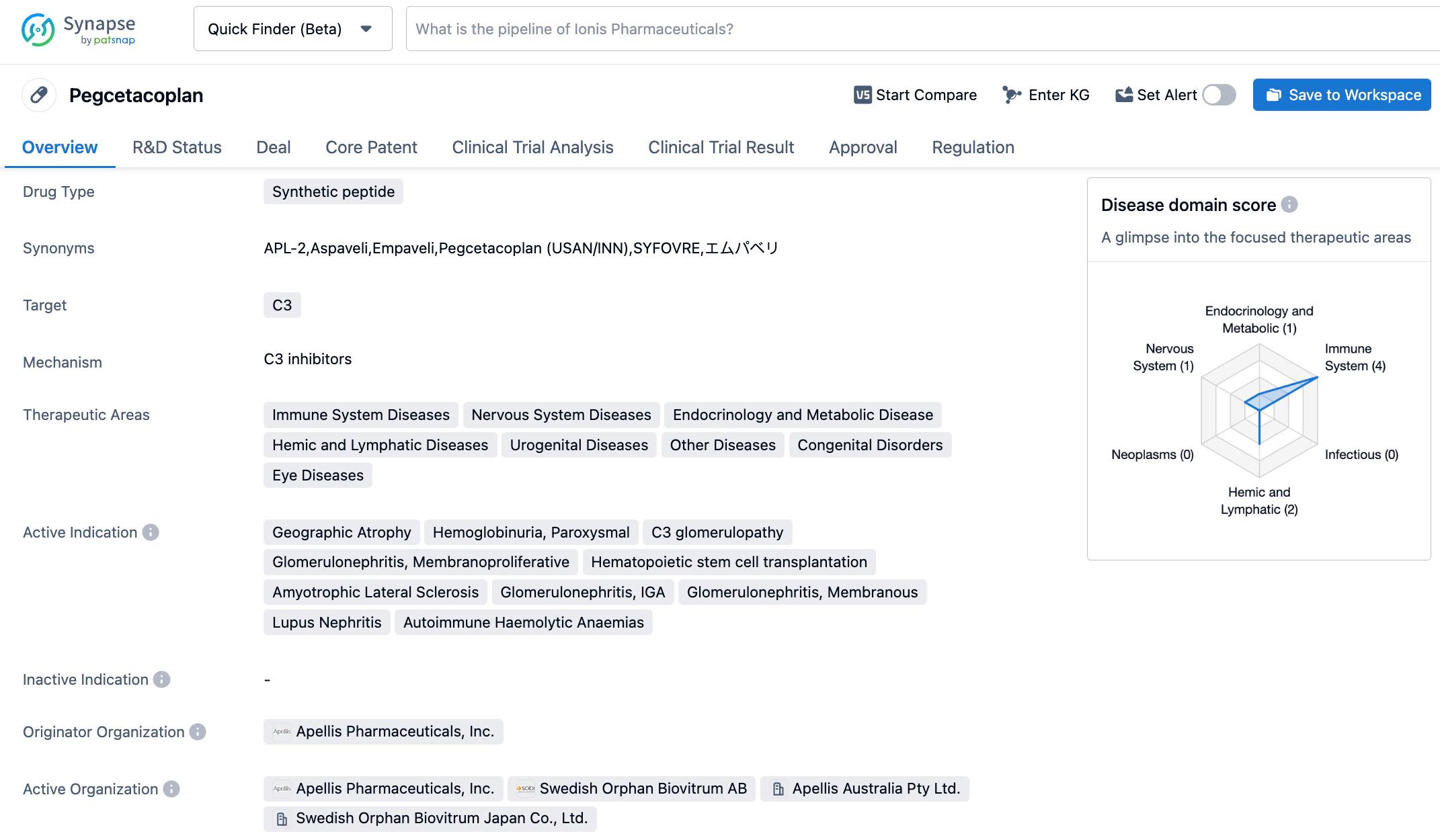
Pegcetacoplan is a cyclic peptide composed of 13 amino acids, which inhibits the formation of C3 convertase, thereby inhibiting the complement pathway at the source. The cyclic peptide has similar affinities and precise target specificity as antibodies, and its small molecular weight allows it to rapidly penetrate tissues. In October 2020, Sobi collaborated with Apellis on Pegcetacoplan. Under the agreement, Sobi obtained the commercial rights to the drug outside the United States, and will pay Apellis $250 million in pre-payment, $915 million in milestone payments, $80 million in R&D expenses, and double-digit royalties on sales. In May 2021, Syfovre received FDA approval for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
The multicenter, randomized double-blind OAKS (included 637 individuals) and DERBY (included 621 individuals) clinical trials evaluated the efficacy and safety of Syfovre compared to a sham injection in patients with GA secondary to AMD. The studies assessed the efficacy of Syfovre in GA patients with monthly and bi-monthly injections, and efficacy was measured by changes in total GA lesion area compared to baseline as measured by fundus autofluorescence. The study's results showed that both every other month and once-monthly treatment with Syfovre showed clinically meaningful reduction in GA lesion growth in over 1200 patients, with effects becoming more apparent over time and safety being adequately demonstrated.
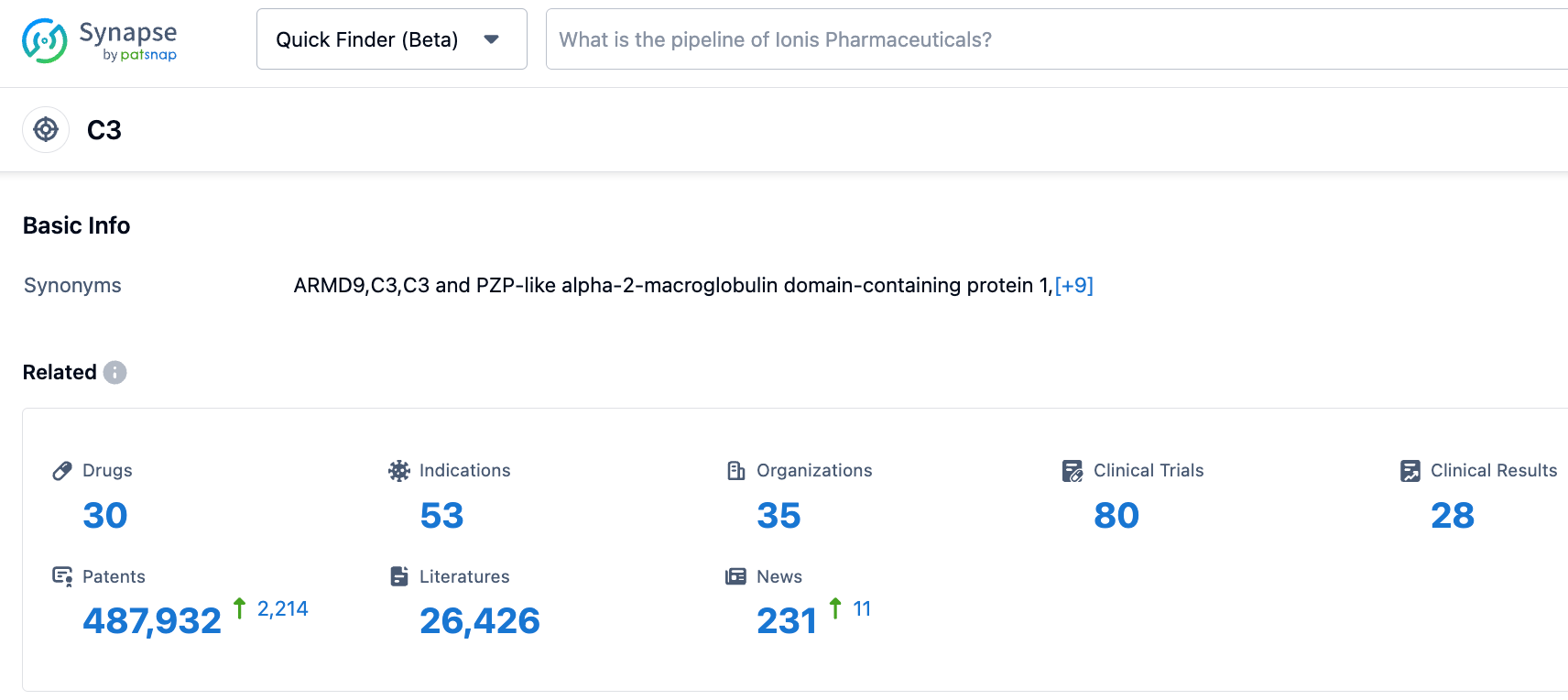
According to the information disclosed by the synapse database, as of October 31, 2023, there are 30 drugs under research for the C3 target, covering 53 indications, involving 35 research institutions, 80 related clinical trials, and as many as 487,942 patents... In addition to Pegcetacoplan, there are several C3-targeting drugs under research worldwide, including peptides (such as ZP 10068), fusion proteins, siRNA (like ARO-C3), bispecific antibodies (like LP-005), and small molecules (like APL-1030). We look forward to Pegcetacoplan standing out in this field.