An In-depth Analysis of Luliconazole's R&D Progress
Luliconazole's R&D Progress
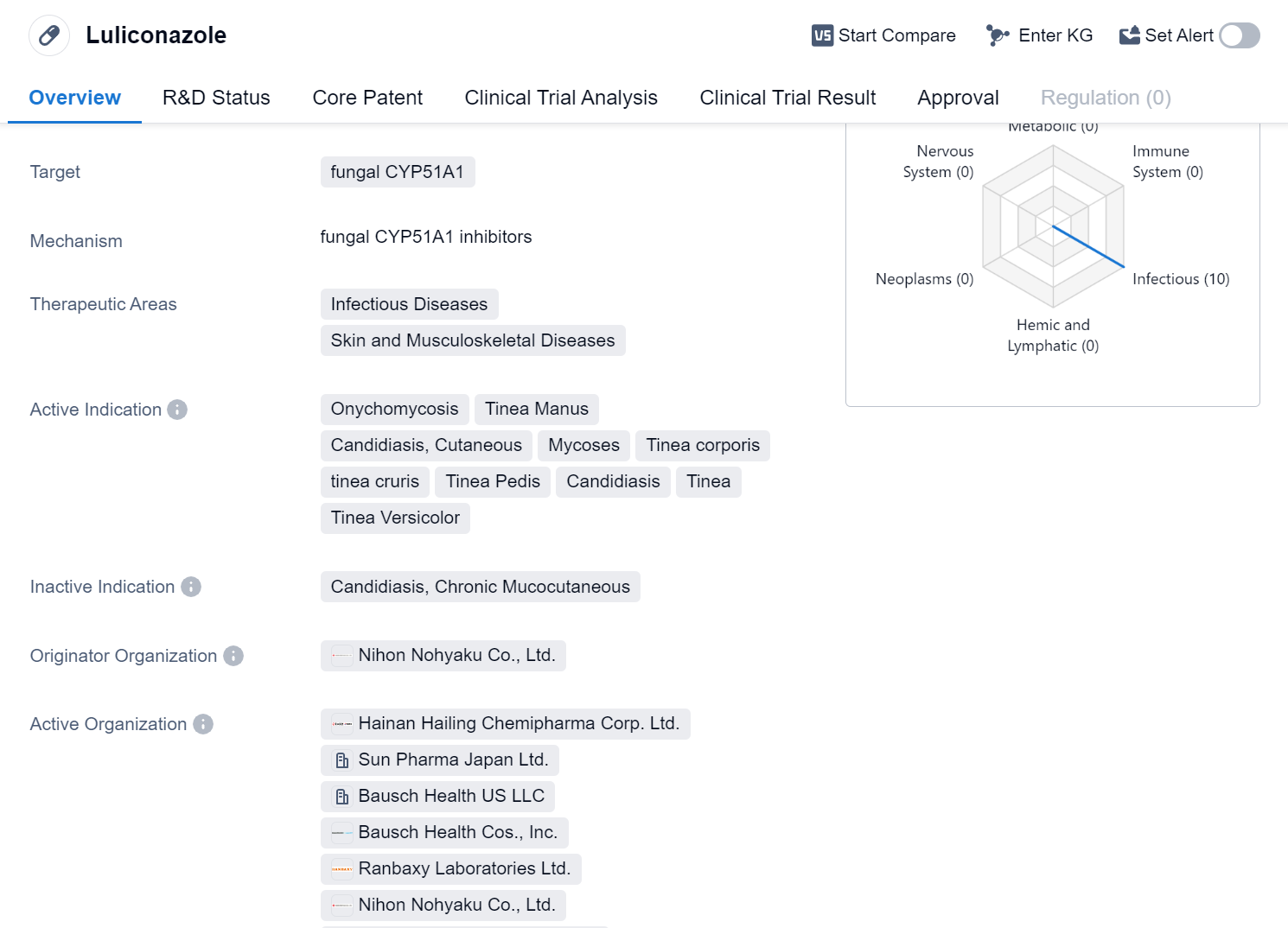
Luliconazole is a small molecule drug that falls under the therapeutic areas of Infectious Diseases and Skin and Musculoskeletal Diseases. It primarily targets fungal CYP51A1, making it effective against various fungal infections. The drug has been approved for use in several indications, including Onychomycosis, Tinea Manus, Candidiasis (Cutaneous), Mycoses, Tinea corporis, tinea cruris, Tinea Pedis, Candidiasis, Tinea, and Tinea Versicolor.
The originator organization of Luliconazole is Nihon Nohyaku Co., Ltd., a pharmaceutical company based in Japan. The drug has received approvals in multiple countries, with its first approval granted in Japan in April 2005. Luliconazole has also obtained approvals in China.
As a small molecule drug, Luliconazole is designed to target specific enzymes involved in fungal infections. By targeting fungal CYP51A1, it disrupts the growth and reproduction of fungi, effectively treating various fungal-related conditions.
The therapeutic areas of Infectious Diseases and Skin and Musculoskeletal Diseases highlight the drug's potential in treating a wide range of fungal-related conditions. Onychomycosis, a fungal infection of the nails, is a common indication for Luliconazole. Additionally, it is approved for treating Tinea Manus (fungal infection of the hands), Candidiasis (Cutaneous), Mycoses (general fungal infections), Tinea corporis (ringworm), tinea cruris (jock itch), Tinea Pedis (athlete's foot), Candidiasis (yeast infection), Tinea (fungal infection), and Tinea Versicolor (a fungal infection causing discolored patches on the skin).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Luliconazole: fungal CYP51A1 inhibitors
From a biomedical perspective, fungal CYP51A1 inhibitors are a type of medication or compound that specifically target and inhibit the activity of the fungal enzyme called CYP51A1. CYP51A1 is an important enzyme involved in the synthesis of ergosterol, a vital component of fungal cell membranes. By inhibiting CYP51A1, these inhibitors disrupt the production of ergosterol, leading to impaired fungal cell membrane integrity and function.
Inhibiting CYP51A1 is an effective strategy for treating fungal infections because it selectively targets the fungal cells while having minimal impact on human cells, as humans use a different enzyme for the synthesis of cholesterol, which is the equivalent of ergosterol in fungal cells. By interfering with ergosterol synthesis, fungal CYP51A1 inhibitors can effectively kill or inhibit the growth of various fungal pathogens, including those causing common infections like candidiasis or aspergillosis.
It's important to note that fungal CYP51A1 inhibitors are typically used in combination with other antifungal agents to maximize their efficacy and reduce the risk of drug resistance. Additionally, the development of these inhibitors plays a crucial role in combating fungal infections and expanding the arsenal of antifungal treatments available to healthcare professionals.
Drug Target R&D Trends for Luliconazole
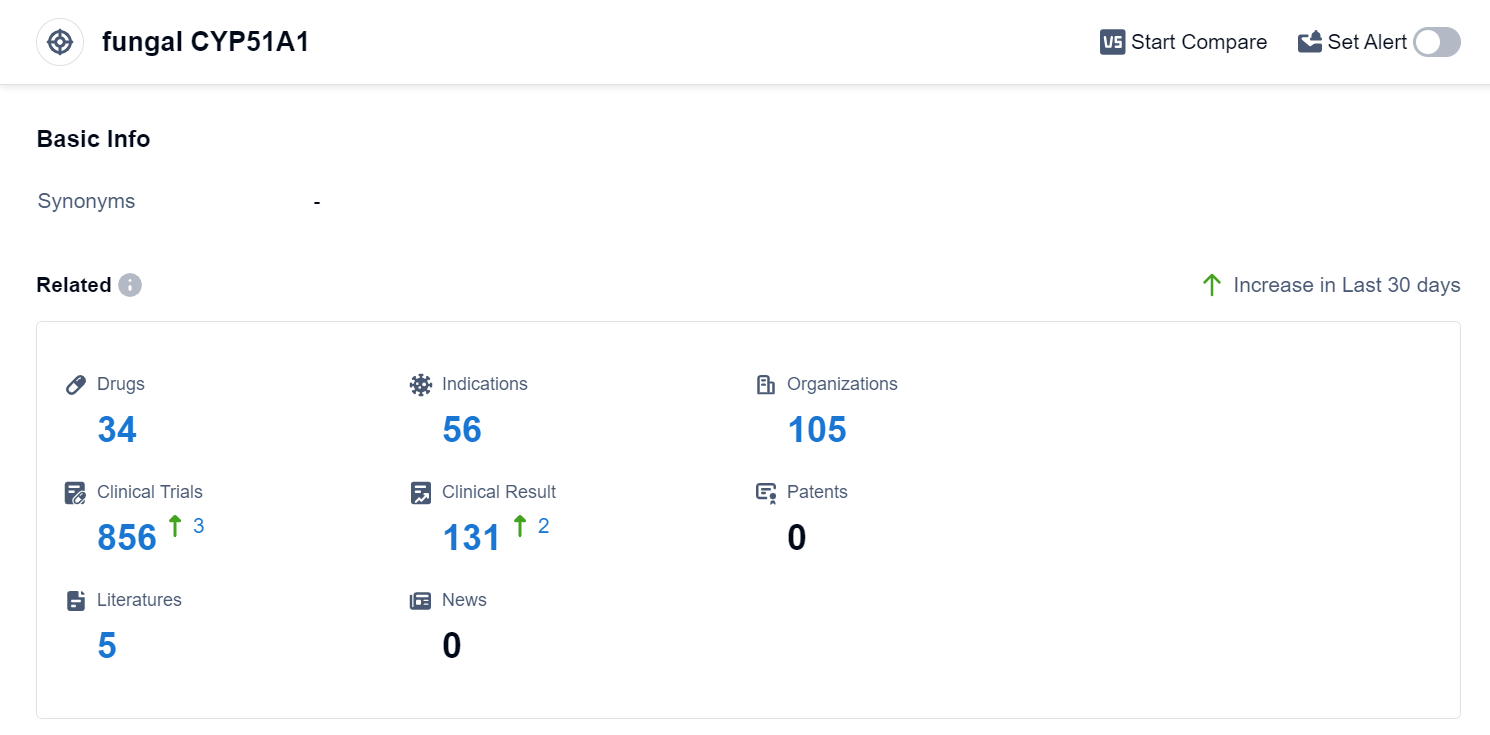
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 34 fungal CYP51A1 drugs worldwide, from 105 organizations, covering 56 indications, and conducting 856 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target fungal CYP51A1 is characterized by multiple companies with drugs in various stages of development. Bausch Health Cos., Inc. has the highest number of drugs in the approved phase, indicating its strong presence in the market. The most common approved indications for drugs targeting fungal CYP51A1 are Mycoses, Tinea Pedis, and Candidiasis. Small molecule drugs are progressing rapidly under this target, with no mention of intense competition from biosimilars. China, the United States, and Japan are the countries/locations with the highest development phase, with China showing significant progress in the approved phase.
Overall, the target fungal CYP51A1 presents a competitive landscape with multiple companies and drug types, indicating a promising future for the development of drugs targeting this specific target.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Luliconazole is a small molecule drug developed by Nihon Nohyaku Co., Ltd. It has been approved for various indications related to fungal infections, making it a valuable asset in the field of biomedicine. Its effectiveness in treating different types of fungal-related conditions and its global approvals highlight its potential in addressing the medical needs of patients suffering from these infections.