Analysis on the Research Progress of CGRP Receptor Antagonist
The CGRP receptor, also known as the calcitonin gene-related peptide receptor, plays a crucial role in the human body. It is a G-protein coupled receptor found in various tissues, including the central and peripheral nervous systems. Activation of the CGRP receptor by the neuropeptide CGRP leads to vasodilation, modulation of pain transmission, and regulation of inflammatory responses. This receptor has gained significant attention in the pharmaceutical industry due to its involvement in migraine pathophysiology. Targeting the CGRP receptor has emerged as a promising therapeutic approach for the treatment of migraines, leading to the development of CGRP receptor antagonists as potential medications.
CGRP Competitive Landscape
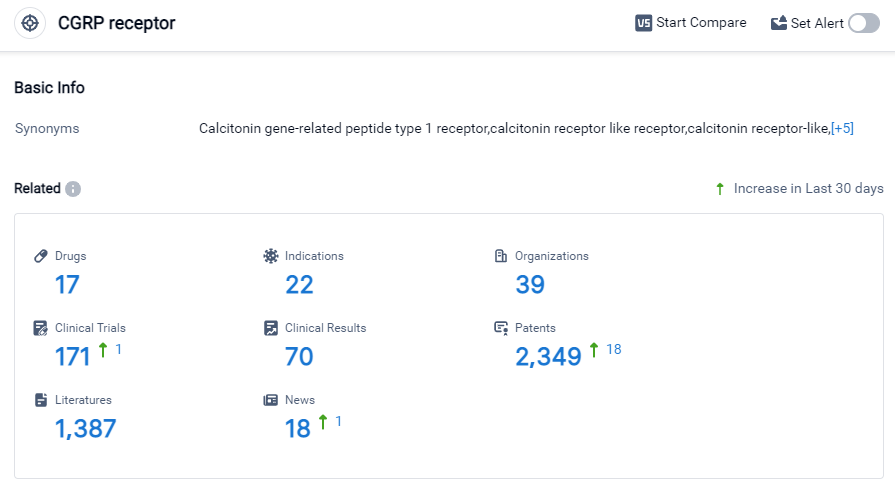
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 17 CGRP receptor drugs worldwide, from 39 organizations, covering 22 indications, and conducting 171 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target CGRP receptor in the pharmaceutical industry reveals that Pfizer Inc., Amgen, Inc., and Novartis AG are the companies growing fastest under this target. Migraine disorders have the highest number of approved drugs, followed by acute migraine, nasal polyps, and chronic sinusitis. Small molecule drugs and monoclonal antibodies are progressing most rapidly under this target. The United States, European Union, and Japan are the countries/locations with the highest development phases. China has also shown progress in the development of drugs targeting the CGRP receptor. The competitive landscape for the target CGRP receptor is intense, with a focus on developing innovative drugs and potential competition from biosimilars. Future development in this area will require further research and analysis to stay competitive in the pharmaceutical industry.
Recently approved for market CGRP antagonist: Rimegepant Sulfate
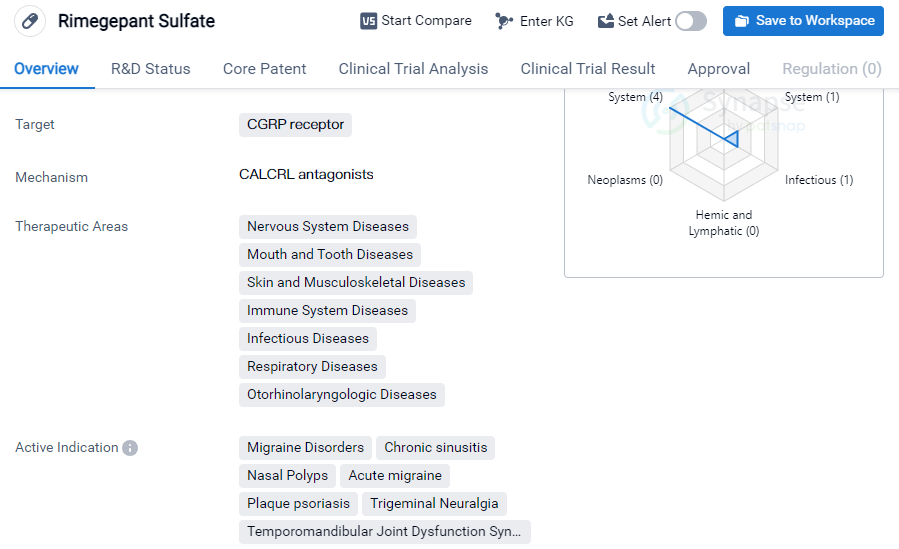
Rimegepant Sulfate is a small molecule drug that targets the CGRP receptor. It has been developed by Biohaven Ltd., a pharmaceutical company specializing in biomedicine. The drug has received approval for use in the treatment of various conditions related to the nervous system, mouth and tooth diseases, skin and musculoskeletal diseases, immune system diseases, infectious diseases, respiratory diseases, and otorhinolaryngologic diseases.
The active indications for Rimegepant Sulfate include migraine disorders, chronic sinusitis, nasal polyps, acute migraine, plaque psoriasis, trigeminal neuralgia, and temporomandibular joint dysfunction syndrome. This indicates that the drug has a broad range of potential applications in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Rimegepant Sulfate has achieved the highest phase of development, which is approval, on a global scale. It received its first approval in the United States in February 2020. This suggests that the drug has undergone rigorous testing and evaluation to ensure its safety and efficacy.
In China, Rimegepant Sulfate is currently in the NDA/BLA phase, indicating that it is undergoing the process of seeking approval in the country.
Overall, Rimegepant Sulfate is a promising drug in the field of biomedicine. Its small molecule structure and targeting of the CGRP receptor make it a potentially effective treatment for various conditions. With its approval in the United States and ongoing efforts for approval in China, the drug has the potential to provide relief to patients suffering from migraine disorders, chronic sinusitis, nasal polyps, and other related conditions. The diverse therapeutic areas it covers further highlight its potential impact in the pharmaceutical industry. As an expert in the pharmaceutical industry, it would be beneficial to closely monitor the progress of Rimegepant Sulfate and its potential impact on patient care and treatment outcomes.
Ubrogepant
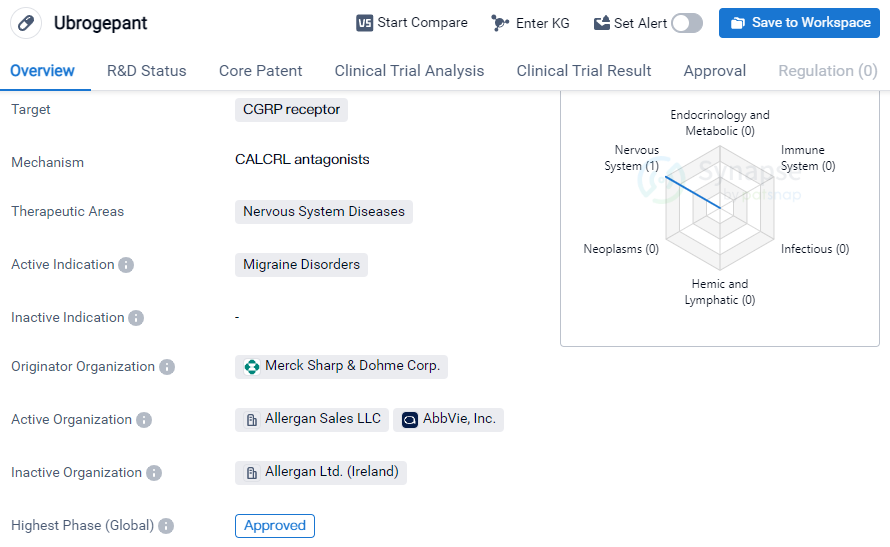
Ubrogepant is a small molecule drug that targets the CGRP receptor and is primarily used in the treatment of migraine disorders. It falls under the therapeutic area of nervous system diseases. The drug was first approved in the United States in December 2019 and is currently in the highest phase of development, which is the approved stage.
Ubrogepant is developed by Merck Sharp & Dohme Corp., a renowned pharmaceutical organization. As a small molecule drug, it is designed to interact with the CGRP receptor, which plays a crucial role in migraine pathophysiology. By targeting this receptor, ubrogepant aims to alleviate the symptoms associated with migraine disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Migraine disorders are a prevalent neurological condition characterized by recurrent headaches, often accompanied by other symptoms such as nausea, vomiting, and sensitivity to light and sound. The approval of ubrogepant provides a new treatment option for individuals suffering from migraines, offering potential relief and improved quality of life. And this approval allows healthcare professionals to prescribe ubrogepant to patients, providing them with a much-needed alternative to existing treatments.
The development and approval of ubrogepant highlight the continuous efforts in the pharmaceutical industry to address unmet medical needs and improve patient outcomes. By specifically targeting the CGRP receptor, ubrogepant offers a targeted approach to migraine treatment, potentially minimizing side effects and enhancing therapeutic efficacy.
In conclusion, ubrogepant is a small molecule drug developed by Merck Sharp & Dohme Corp. that targets the CGRP receptor for the treatment of migraine disorders. Its approval in the United States in December 2019 signifies its safety and efficacy in alleviating migraine symptoms. This development represents a significant advancement in the field of biomedicine, providing healthcare professionals with a new treatment option to improve the lives of individuals suffering from migraines.
The earliest approved CGRP receptor antagonist for market launch: Erenumab
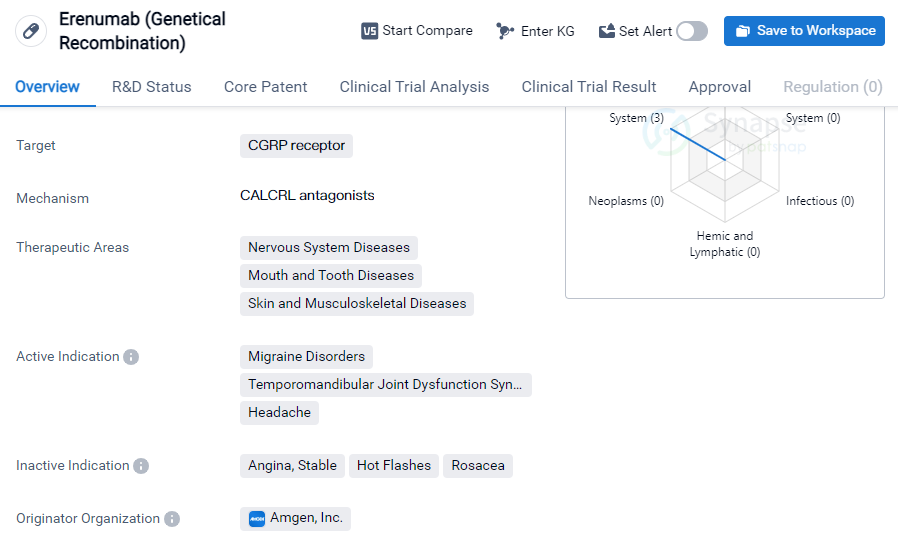
Erenumab (Genetical Recombination) is a monoclonal antibody drug that targets the CGRP receptor. It is primarily used in the treatment of various nervous system diseases, mouth and tooth diseases, as well as skin and musculoskeletal diseases. The active indications for this drug include migraine disorders, temporomandibular joint dysfunction syndrome, and headaches.
The drug was developed by Amgen, Inc., a renowned pharmaceutical company. It has received approval for use in the highest phase globally. The first approval for Erenumab (Genetical Recombination) was granted in May 2018 in the United States.
As a monoclonal antibody, Erenumab (Genetical Recombination) works by targeting the CGRP receptor. CGRP, or calcitonin gene-related peptide, is a neuropeptide involved in the transmission of pain signals. By blocking the CGRP receptor, this drug aims to reduce the frequency and severity of migraines, alleviate symptoms associated with temporomandibular joint dysfunction syndrome, and provide relief from headaches.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The approval of Erenumab (Genetical Recombination) marks a significant advancement in the treatment of these conditions. Migraine disorders, in particular, affect a substantial portion of the population and can significantly impact the quality of life for those affected. The introduction of a targeted therapy like Erenumab (Genetical Recombination) offers new hope for patients who have previously struggled to find effective treatment options.
The approval of this drug in the global markets highlights its potential to address the unmet medical needs of patients worldwide. The fact that it has reached the highest phase of approval indicates that it has undergone rigorous testing and demonstrated its safety and efficacy.
In summary, Erenumab (Genetical Recombination) is a monoclonal antibody drug developed by Amgen, Inc. It targets the CGRP receptor and is approved for the treatment of various nervous system diseases, mouth and tooth diseases, and skin and musculoskeletal diseases. Its active indications include migraine disorders, temporomandibular joint dysfunction syndrome, and headaches. The drug has received approval in the United States and China, marking a significant advancement in the field of biomedicine.