An Overview of Incyte’s Drug Pipeline | Therapeutic Areas
Incyte Corp. founded in 2002 in Wilmington, Delaware, USA, is a global biopharmaceutical company dedicated to the discovery and development of new drugs (focusing on tumors, inflammation, autoimmunity). The scientific research team consists of more than 600 scientists, chemists and biologists engaged in immunology research, with > 1400 employees, and branches in the United States, Europe and Japan. As early as the second half of 2011, the company obtained the marketing approval of Ruxolitinib from the US FDA, which is a very representative milestone for the company. In 2019, the company announced a cooperation license agreement with Zai Lab, Ltd., a Chinese company, to develop the PD-1 monoclonal antibody INCMGA-0012 in Greater China and strive to commercialize it.
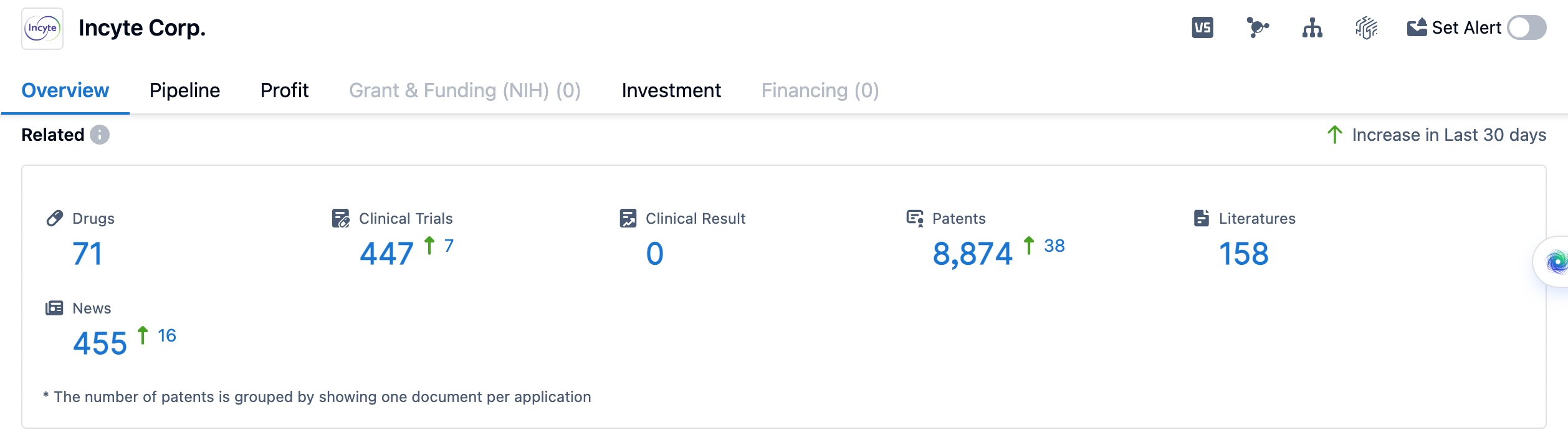
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Incyte.
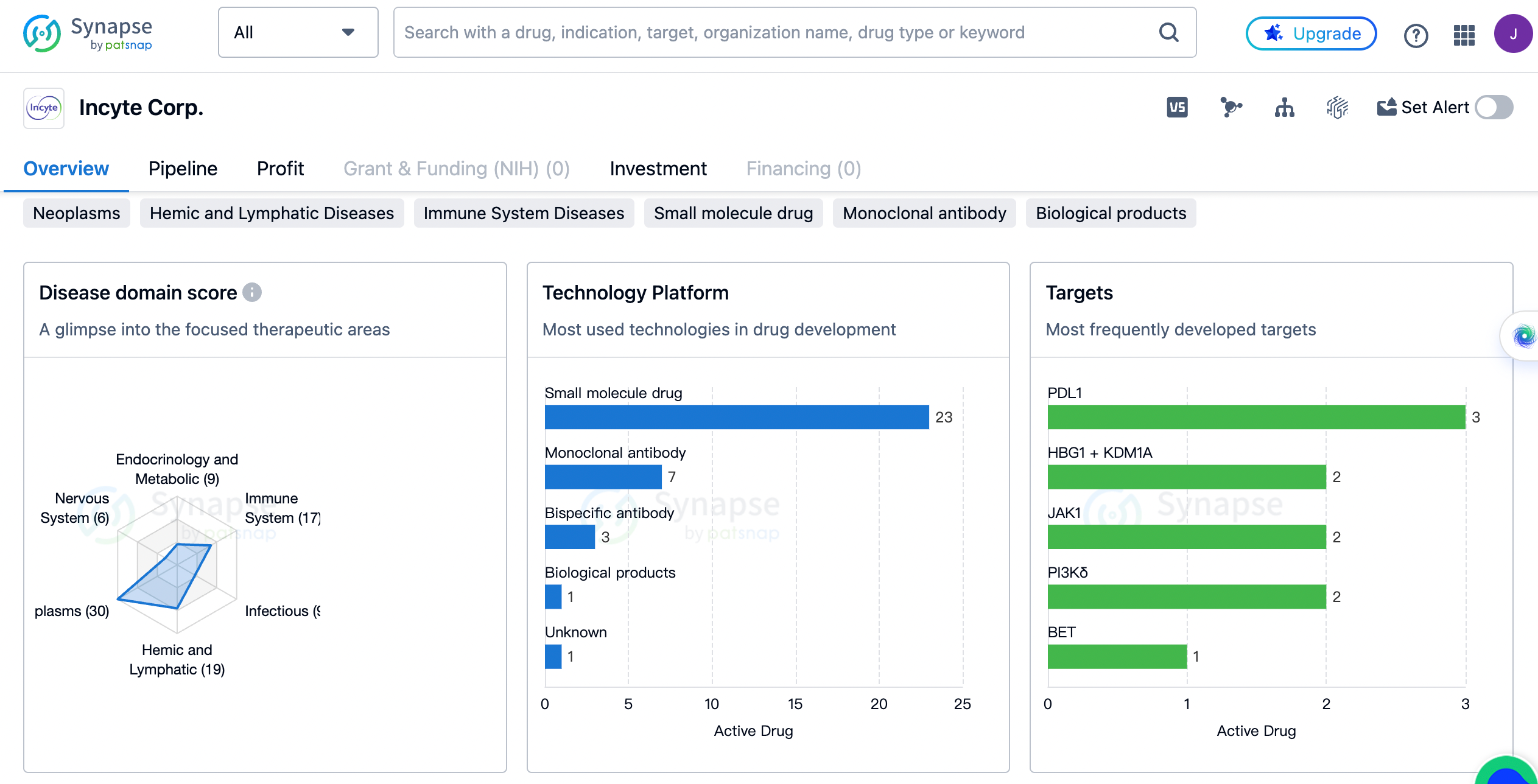
Incyte Corp. has developed drugs for a wide range of therapeutic areas. The highest number of drugs, 30 in total, are focused on the treatment of neoplasms, which are abnormal growths of tissue commonly referred to as tumors. This indicates that Incyte Corp. has a strong emphasis on oncology and is actively involved in developing treatments for different types of cancers.
Following neoplasms, the next highest number of drugs, 19 in total, are dedicated to hemic and lymphatic diseases. These diseases affect the blood and lymphatic systems, including conditions such as leukemia and lymphoma. Incyte Corp.'s focus on this therapeutic area suggests a commitment to addressing disorders related to blood and lymphatic systems.
The company has also developed drugs for immune system diseases, skin and musculoskeletal diseases, respiratory diseases, digestive system disorders, and urogenital diseases. This demonstrates a broad approach to addressing various medical conditions affecting different parts of the body.
In terms of drug count, infectious diseases, endocrinology and metabolic diseases, and nervous system diseases have a relatively lower representation in Incyte Corp.'s portfolio. However, it is important to note that the number of drugs developed does not necessarily reflect the significance or impact of the therapeutic area.
The most frequently developed targets by Incyte
Let’s take a look on the most frequently developed targets by Incyte Corp. and their corresponding drug counts. PDL1, a protein involved in regulating the immune system, is the most frequently targeted with three drugs developed. HBG1 + KDM1A, JAK1, and PI3Kδ are the next most targeted with two drugs each. These targets are associated with various diseases, including cancer and autoimmune disorders.
Other targets with a single drug developed by Incyte Corp. include BET, CDK2, CD47, CCR2, TIM3, 4-1BB + PDL1, ALK2, BRD4, PD-1, LAG3, FGFR1 + FGFR2 + FGFR3, H4 receptor, IL-2Rβ, CSF-1R, OX40, and PI3Kγ. These targets represent a diverse range of proteins and receptors involved in different disease pathways.
An overview of Incyte's pipeline
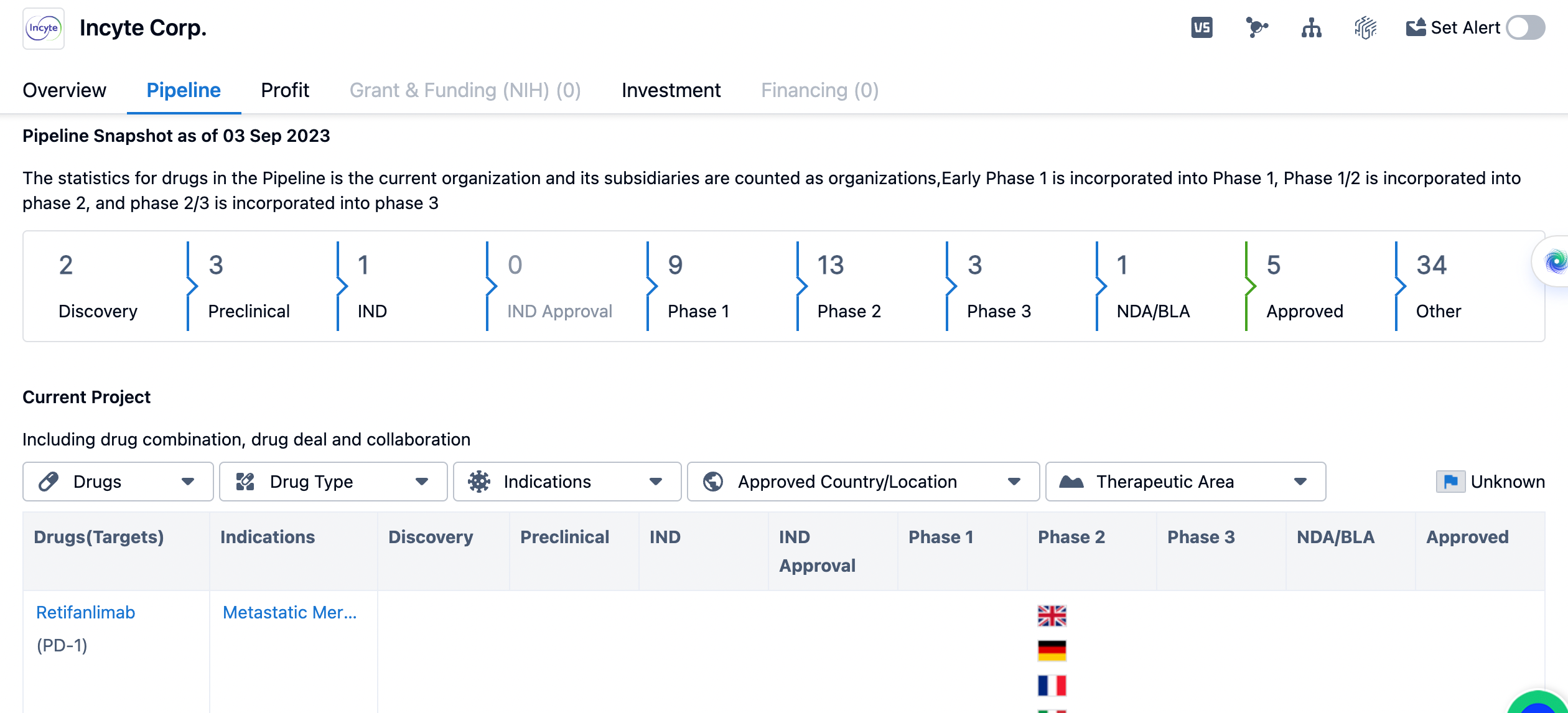
Here’s an overview of Incyte Corp.'s pipeline until September, 2023. The pipeline consists of drugs at various stages of development, starting from the discovery phase to approved drugs. As of the given date, Incyte Corp. has two drugs in the discovery phase, three in the preclinical phase, and one in the IND (Investigational New Drug) phase. The IND approval phase does not have any drugs listed. In terms of clinical development, Incyte Corp. has nine drugs in Phase 1, which involves testing the safety and dosage of the drug in a small group of healthy volunteers or patients. Thirteen drugs are in Phase 2, where the drug's effectiveness and side effects are evaluated in a larger group of patients. Three drugs have reached Phase 3, which involves large-scale clinical trials to confirm the drug's efficacy and monitor its side effects in a larger patient population.
One drug has reached the NDA/BLA (New Drug Application/Biologics License Application) stage, indicating that it has been submitted to regulatory authorities for approval. Five drugs have been approved, suggesting that Incyte Corp. has successfully brought these drugs to market. Additionally, there are 34 drugs listed under "Other," which could include drugs in earlier stages of development or those that have not progressed to clinical trials yet.
In summary, Incyte Corp. is a pharmaceutical organization that has been operating since 1991. The company is based in Delaware, United States, and focuses on the field of biomedicine. Incyte Corp. has developed drugs targeting various therapeutic areas, with a particular emphasis on neoplasms and hemic and lymphatic diseases. The company has also targeted immune system diseases, skin and musculoskeletal diseases, respiratory diseases, digestive system disorders, and urogenital diseases. In terms of drug targets, Incyte Corp. has developed drugs targeting proteins and receptors such as PDL1 and PI3Kδ. These targets are associated with a range of diseases, including cancer and autoimmune disorders. Incyte Corp.'s pipeline includes drugs at different stages of development, from discovery to approved drugs. The company has a significant number of drugs in Phase 1 and Phase 2, indicating active clinical development. Overall, Incyte Corp. demonstrates a commitment to developing innovative treatments for various medical conditions.