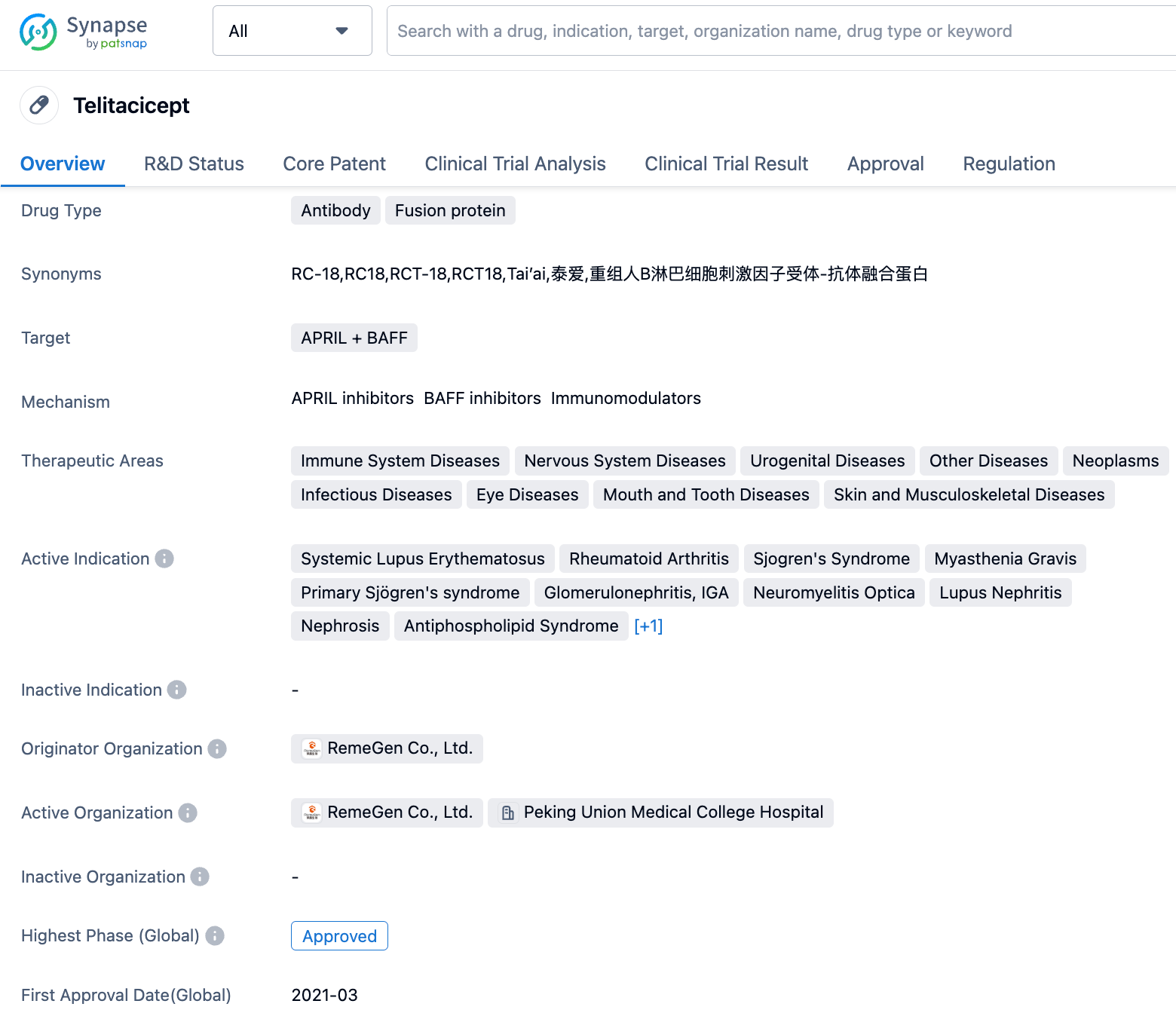
RemeGen's Telitacicept new indication application for the treatment of Rheumatoid Arthritis has been accepted by the CDE
On September 7, 2023, the new indication for the injectable drug Telitacicept developed by RemeGen was accepted by the Center for Drug Evaluation (CDE) for the treatment of rheumatoid arthritis (RA). This is the second indication for Telitacicept to be listed for market approval following the approval of its indication for systemic lupus erythematosus in March 2021.
Telitacicept is a novel fusion protein developed by RemeGen for the treatment of autoimmune diseases. It is composed of the extracellular domain of human Transmembrane Activator and CAML Interactor (TACI) receptor and the crystallizable fragment (Fc) regions of human immunoglobulin G (IgG). Telitacicept can inhibit both BLyS and APRIL simultaneously, effectively reducing B-cell mediated autoimmune responses, which are associated with many autoimmune diseases. On March 9, 2021, China's NMPA approved Telitacicept for conditional marketing for the treatment of adults with active, autoantibody-positive systemic lupus erythematosus (SLE). In October 2022, Telitacicept received orphan drug designation from the U.S. FDA for the treatment of myasthenia gravis. Telitacicept is currently conducting phase III clinical trials in the U.S. for SLE indication and is expected to be completed by 2024.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The clinical study concerning rheumatoid arthritis was a randomized, double-blind, placebo-controlled, multicenter phase III clinical trial involving 479 patients. According to the full analysis set (FAS) results, at week 24, the ACR20 response rate in patients receiving Telitacicept (160 mg) combined with methotrexate was significantly higher than in patients receiving methotrexate alone, achieving the primary efficacy endpoint of the trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
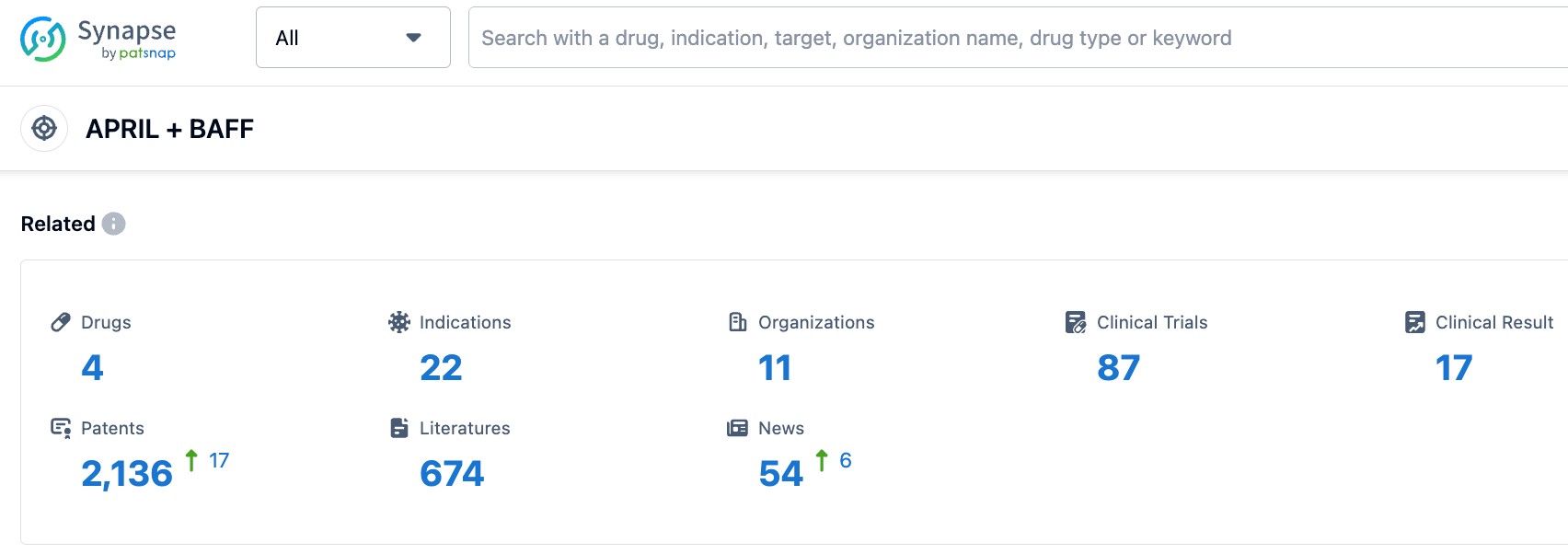
According to the information disclosed by the Synapse Database, as of September 8, 2023, there were 4 investigational drugs for the BLyS/APRIL target, including 22 indications, 11 research institutions, and involved 85 clinical trials and up to 2131 patents. Apart from SLE and RA, phase III clinical studies of Telitacicept for the treatment of IgA nephropathy, neuromyelitis optica spectrum disorders, primary Sjögren's syndrome, myasthenia gravis and multiple sclerosis are underway, showcasing its wide prospects in treating B cell related diseases.