Analysis on the Clinical Research Progress of ERK Inhibitor
ERK, also known as Extracellular Signal-Regulated Kinase, is a crucial protein kinase that plays a significant role in various cellular processes within the human body. It is a part of the mitogen-activated protein kinase (MAPK) signaling pathway, which regulates cell growth, proliferation, differentiation, and survival. ERK is activated by external signals such as growth factors, hormones, and stress, and it subsequently phosphorylates and activates downstream targets, including transcription factors and other kinases. Dysregulation of ERK signaling has been implicated in numerous diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases.
Expression of ERK proteins is almost ubiquitous in all mammals. So far, two subtypes of ERK proteins have been identified: ERK1 and ERK2, which have a high homology of up to 84% in amino acid sequence. For instance, human ERK is composed of 379 amino acid residues for ERK1 and 360 residues for ERK2. Currently, up to hundreds of known ERK substrates are mainly distributed in the cell membrane, cytoplasm, and cell nucleus. Cytoplasmic substrates for ERK1/2 include proteins like RSK family protein kinases, phosphoprotein phosphatases, cAMP phosphodiesterase, cytoplasmic phospholipase A2, and cytoskeletal proteins. Meanwhile, the substrates for ERK1/2 in the nucleus are mostly transcription factors, predominantly the TCF family. Through regulation of these various substrates, ERK plays differing biological roles. As the only key downstream kinase in the MAPK signaling pathway, ERK kinase holds potential to overcome acquired resistance or non-druggability of upstream kinases, providing a possible treatment pathway for a significant population of patients.
ERK inhibitors, therefore, have attracted increasing attention in recent years. This attention is largely due to the emergence of resistance mechanisms induced by inhibitors of upstream components in ERK, and ERK's crucial role as the final component of the pathway, directly inducing transcription of multiple downstream substrates. Directly blocking ERK can inhibit abnormally activated pathway signals at the most crucial points in the pathway.
Additionally, numerous studies have observed recovery in cancer patients who were previously resistant to the inhibitory effects of EGFR, RAF, and MEK inhibitors, due to reactivation of the pathway. This reactivation may be a result of amplification or mutation of certain components in the pathway. The combination of ERK inhibitors with inhibitors of its upstream components can overcome this resistance. Research has shown that whether it is the resistance to targeted drugs for a single upstream component of the pathway, or to multi-component targeted drugs, combining ERK inhibitors with inhibitors of other components significantly inhibits the pathway signal and has a therapeutic effect.
ERK Competitive Landscape
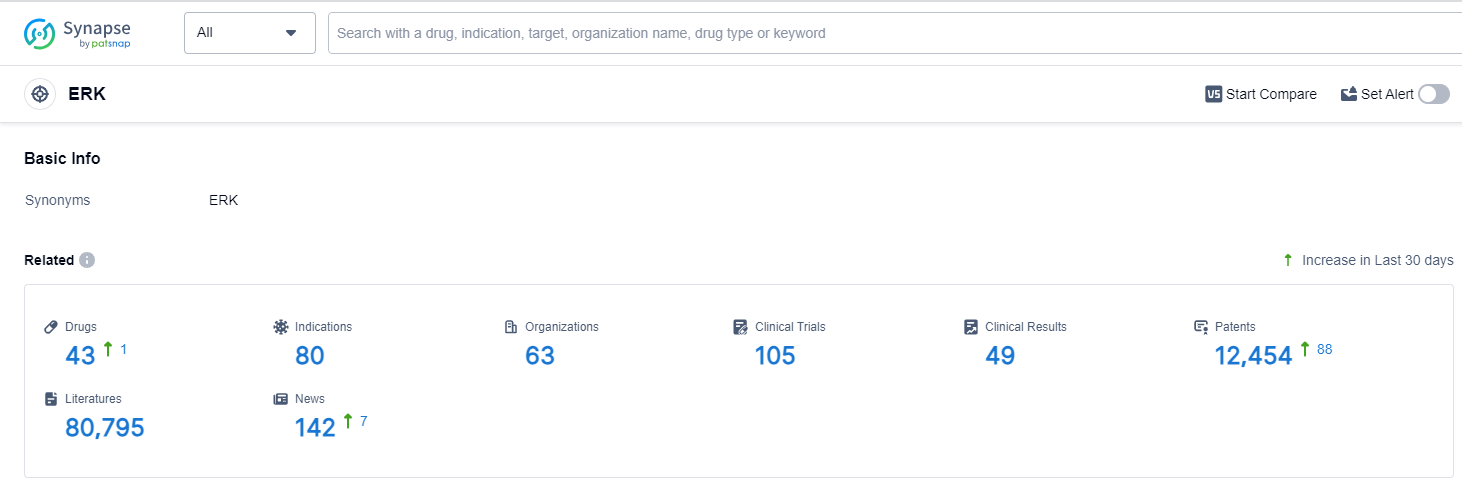
According to Patsnap Synapse, as of 13 Oct 2023, there are a total of 43 ERK drugs worldwide, from 63 organizations, covering 80 indications, and conducting 105 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the data provided, the current competitive landscape of the target ERK in the pharmaceutical industry is characterized by the presence of multiple companies actively pursuing R&D in various stages of development. The highest stage of development is Phase 3, indicating the advancement of drugs towards potential approval. Indications such as Alzheimer Disease, Gingival Diseases, and various types of cancers have seen drug approvals under the target ERK. Small molecule drugs, herbal medicine, and recombinant proteins are the most rapidly progressing drug types, indicating intense competition. The United States, European Union, and China are the leading countries/locations in the development of drugs targeting ERK. China has shown significant progress in recent years. Overall, the target ERK presents a promising area for future development in the pharmaceutical industry.
New Type of Antitumor Drug: HRS-2543
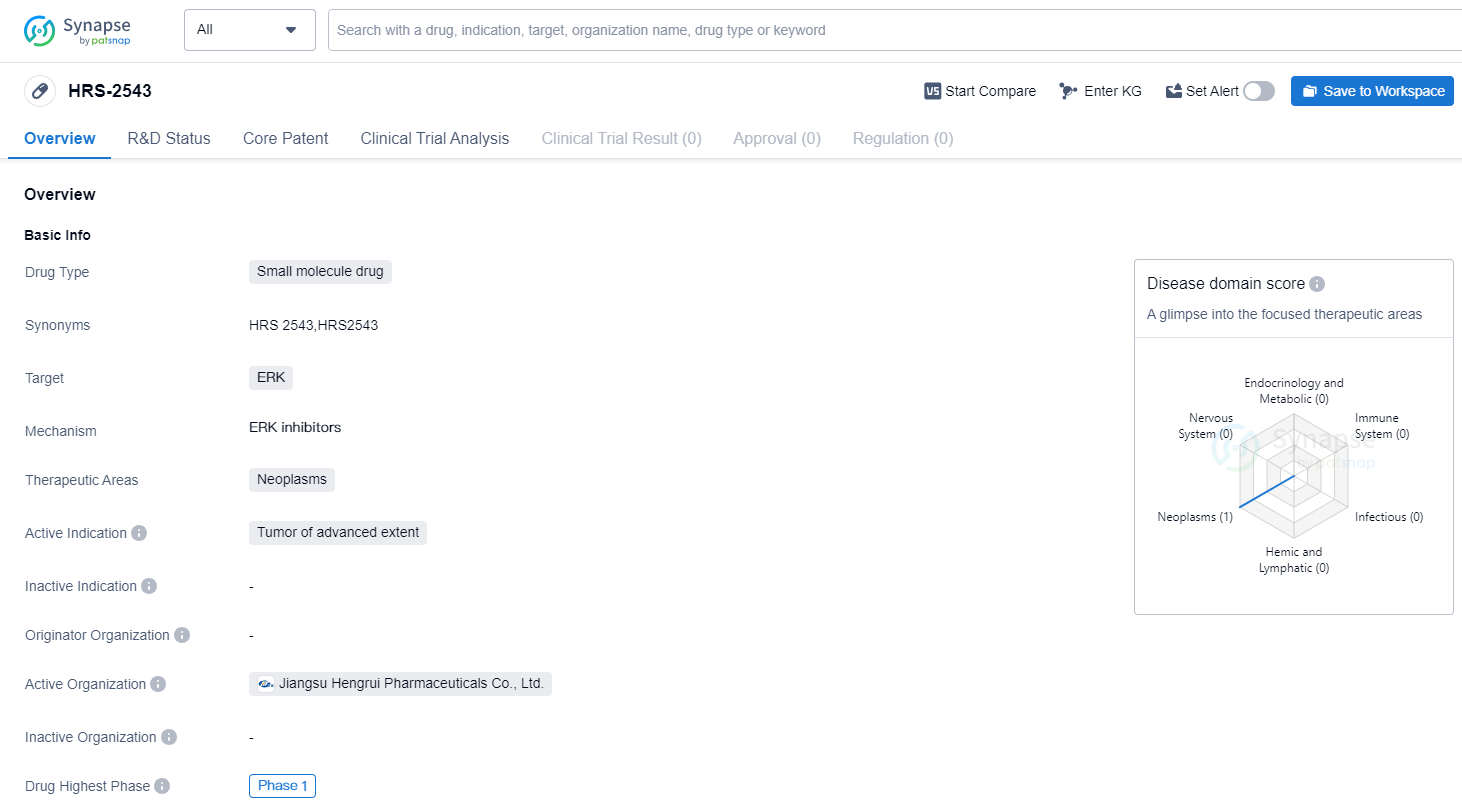
HRS-2543 is a small molecule drug that targets ERK, a protein involved in cell signaling pathways. It is primarily being developed for the treatment of neoplasms, specifically tumors of advanced extent.
Currently, HRS-2543 is in Phase 1 of clinical development, both globally and in China. Phase 1 is the initial stage of testing in humans, where the drug's safety, dosage, and potential side effects are evaluated. This suggests that HRS-2543 is still in the early stages of development and has not yet progressed to larger-scale trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The choice of targeting ERK in the development of HRS-2543 is significant, as ERK is known to play a crucial role in cell proliferation, differentiation, and survival. Abnormal activation of ERK has been observed in various types of cancers, making it an attractive target for therapeutic intervention. By inhibiting ERK, HRS-2543 aims to disrupt the signaling pathways that promote tumor growth and progression.
Neoplasms, or tumors, are abnormal growths of cells that can be either benign or malignant. Malignant tumors, such as advanced-stage cancers, have the potential to invade nearby tissues and spread to other parts of the body. The fact that HRS-2543 is specifically indicated for tumors of advanced extent suggests that it may be intended for patients with more aggressive and advanced forms of cancer.
While the information provided does not offer any specific details about the drug's mechanism of action or its potential efficacy, it does provide a basic understanding of HRS-2543's development stage, target, and therapeutic area. As a small molecule drug targeting ERK, HRS-2543 holds promise for the treatment of neoplasms, particularly advanced-stage tumors. However, further clinical trials and research are needed to determine its safety and effectiveness in treating these conditions.
Expected to Solve Drug Resistance Problem - ERK Inhibitor HMPL-295S1
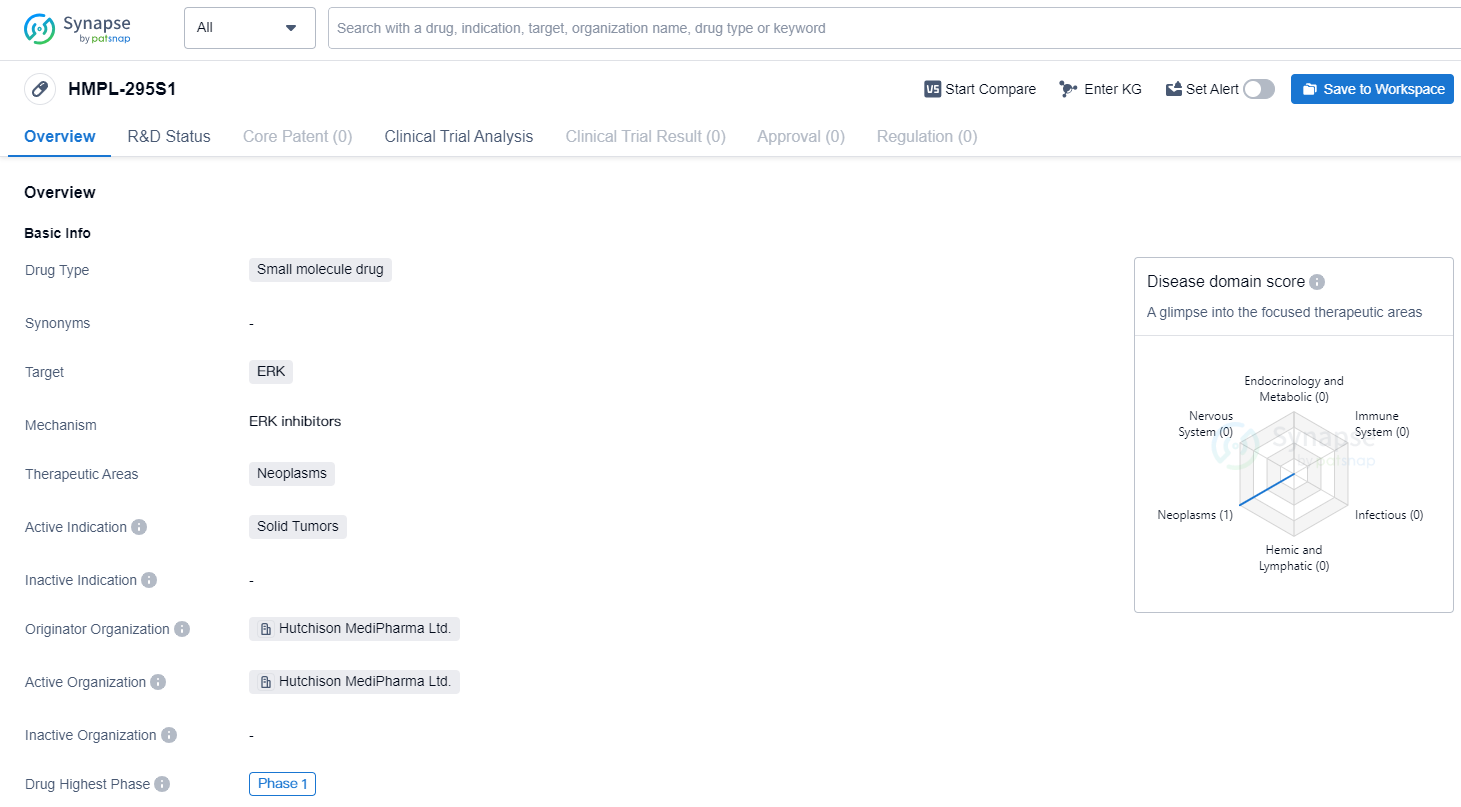
HMPL-295S1 is a small molecule drug developed by Hutchison MediPharma Ltd. It falls under the therapeutic area of neoplasms, specifically targeting ERK. The drug is currently in the highest phase of clinical development, which is Phase 1, both globally and in China.
Being a small molecule drug, HMPL-295S1 is characterized by its relatively low molecular weight and ability to penetrate cell membranes. This makes it a promising candidate for the treatment of solid tumors, which are characterized by abnormal cell growth and division. By targeting ERK, the drug aims to inhibit the signaling pathway involved in tumor growth and proliferation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Hutchison MediPharma Ltd. is the originator organization behind HMPL-295S1. As the developer of the drug, they are responsible for its research, development, and eventual commercialization. The fact that the drug has reached Phase 1 in both global and Chinese clinical trials indicates that it has undergone initial safety testing and is now being evaluated for its efficacy and dosage in human subjects.
Phase 1 trials are typically conducted on a small number of healthy volunteers or patients to assess the drug's safety, dosage, and potential side effects. This phase is crucial in determining the drug's tolerability and establishing a safe dosage range for further development. The fact that HMPL-295S1 has reached Phase 1 suggests that it has shown promising results in preclinical studies and has met the necessary criteria to progress to human trials.
In summary, HMPL-295S1 is a small molecule drug developed by Hutchison MediPharma Ltd. It targets ERK and is being developed for the treatment of solid tumors. Currently in Phase 1 of clinical development globally and in China, the drug has shown potential in preclinical studies and is now being evaluated for its safety and efficacy in human subjects.