Analysis on the Clinical Research Progress of Factor Xa Inhibitor
Factor Xa, a trypsin-like serine protease, is situated at the critical juncture between the intrinsic and extrinsic pathways, catalyzing the conversion of prothrombin to thrombin, and hence plays a pivotal role in the final common pathway of the cascade and has become an important target in the discovery and development of new anticoagulants. Factor Xa is a key protease of the coagulation pathway whose activity is known to be in part modulated by binding to factor Va and sodium ions.
Blood coagulation involves a complex cascade of enzymatic reactions, ultimately generating fibrin, the basis of all blood clots. This cascade is comprised of two arms, the intrinsic and extrinsic pathways which converge at factor Xa to form the common pathway. Factor Xa activates prothrombin to thrombin, which in turn catalyzes the conversion of fibrinogen to fibrin.
Due to its significance in the coagulation process, Factor Xa has become an important target for pharmaceutical interventions, with the development of Factor Xa inhibitors that can help prevent and treat thrombotic disorders.
Factor Xa Competitive Landscape
According to Patsnap Synapse, as of 13 Oct 2023, there are a total of 126 factor Xa drugs worldwide, from 124 organizations, covering 66 indications, and conducting 1448 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.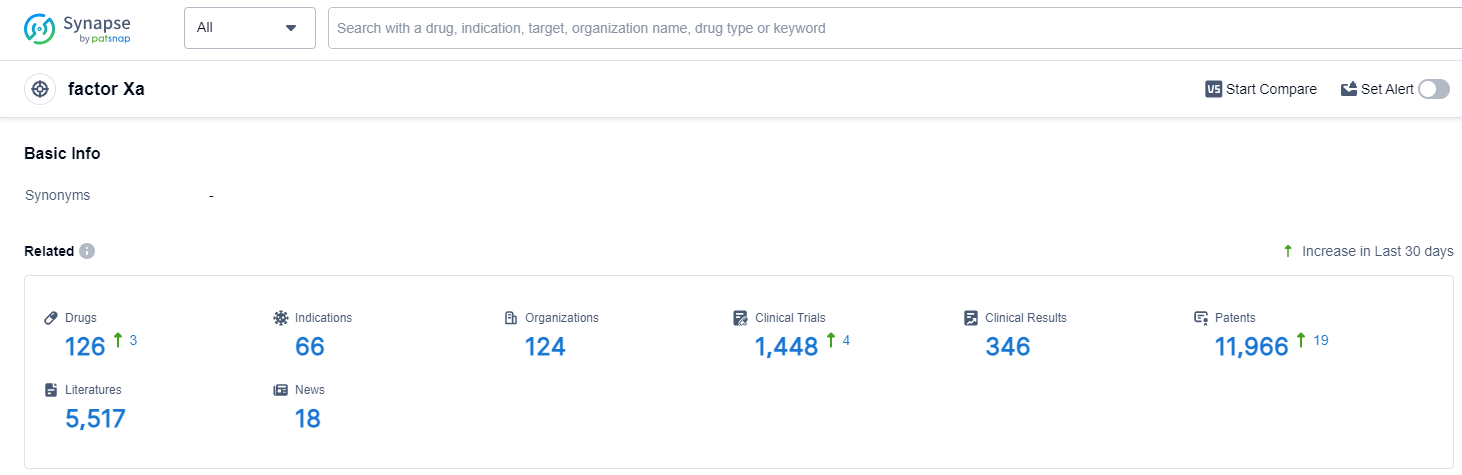
Based on the analysis of the data provided, Pfizer Inc. is the company with the highest number of drugs in the approved phase under the target factor Xa. The indications with the most approved drugs are Venous Thrombosis, Venous Thromboembolism, and Pulmonary Embolism. Small molecule drugs and chemical drugs are progressing most rapidly under the current targets. The European Union, the United States, and Japan are the countries/locations developing fastest under the target factor Xa. China also shows progress with drugs in the approved and preclinical phases. Overall, the competitive landscape for target factor Xa is dynamic, with multiple companies and drug types involved in the research and development of drugs for various indications. The future development of target factor Xa is expected to continue with a focus on improving treatment options for thrombotic and cardiovascular conditions.
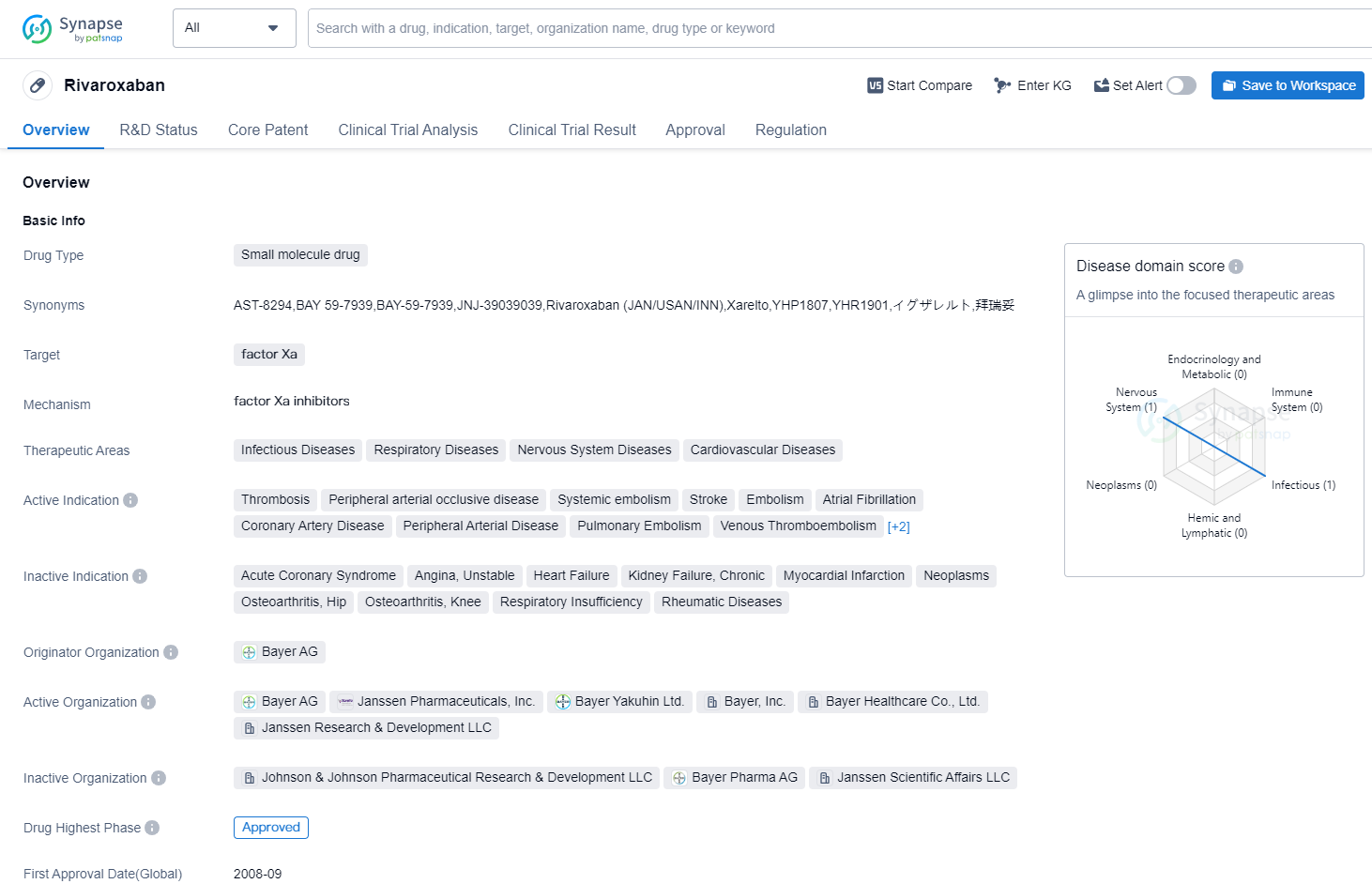
Key drug:Rivaroxaban
Rivaroxaban is a small molecule drug that targets factor Xa, an enzyme involved in blood clotting. It has been approved for use in various therapeutic areas including infectious diseases, respiratory diseases, nervous system diseases, and cardiovascular diseases. The drug is indicated for the treatment of thrombosis, peripheral arterial occlusive disease, systemic embolism, stroke, embolism, atrial fibrillation, coronary artery disease, peripheral arterial disease, pulmonary embolism, venous thromboembolism, venous thrombosis, and even COVID-19.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Rivaroxaban is Bayer AG, a multinational pharmaceutical company. The drug has reached the highest phase of approval both globally and in China. It received its first approval in Canada in September 2008, marking its initial entry into the market.
In terms of regulation, Rivaroxaban has undergone priority review and fast track processes. These regulatory pathways expedite the approval process for drugs that address unmet medical needs or provide significant advancements in treatment options.
Rivaroxaban's approval for multiple therapeutic areas and indications highlights its versatility and potential impact in various medical conditions. Its ability to target factor Xa makes it a valuable tool in preventing and treating blood clot-related disorders. The drug's approval in China and other countries further demonstrates its global recognition and acceptance.
As an expert in the pharmaceutical industry, analyzing the information provided can help identify the drug's market potential and opportunities for business development. The wide range of therapeutic areas and indications suggests a broad patient population that could benefit from Rivaroxaban. Additionally, the drug's fast track and priority review designations indicate regulatory support and potential for accelerated market entry.
Overall, Rivaroxaban's approval, target specificity, and broad therapeutic applications position it as a significant player in the pharmaceutical industry. Its originator organization, Bayer AG, has successfully developed and brought this drug to market, showcasing their expertise in the field. As the drug continues to be used in various medical conditions, further research and development may uncover additional indications and expand its market reach.
Betrixaban
Betrixaban is a small molecule drug that targets factor Xa and is primarily used in the treatment of cardiovascular diseases. Specifically, it is indicated for the treatment of venous thromboembolism. The drug was developed by Millennium Pharmaceuticals, Inc., and it reached the highest phase of clinical trials, which is Phase 3.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Betrixaban received its first approval globally in June 2017, with the United States being the first country to approve it. The drug was granted Fast Track designation, which is a regulatory process designed to expedite the development and review of drugs that address unmet medical needs.
As a small molecule drug, Betrixaban is likely to have a well-defined chemical structure and can be easily synthesized in a laboratory setting. Its target, factor Xa, is an enzyme involved in the blood clotting process. By inhibiting factor Xa, Betrixaban can prevent the formation of blood clots, particularly in the veins.
Cardiovascular diseases, including venous thromboembolism, are a significant health concern worldwide. Venous thromboembolism refers to the formation of blood clots in the veins, which can lead to serious complications such as pulmonary embolism. Betrixaban's approval for this indication suggests that it has demonstrated efficacy and safety in clinical trials.
The fact that Betrixaban reached Phase 3 trials indicates that it has undergone extensive testing in humans to evaluate its effectiveness and safety profile. This phase typically involves large-scale studies involving thousands of patients. The successful completion of Phase 3 trials is a crucial step towards obtaining regulatory approval.
The Fast Track designation granted to Betrixaban suggests that there is a recognized need for new treatments in the field of cardiovascular diseases, particularly for venous thromboembolism. This designation allows for a more streamlined regulatory process, potentially accelerating the availability of the drug to patients.
In summary, Betrixaban is a small molecule drug developed by Millennium Pharmaceuticals, Inc. It targets factor Xa and is indicated for the treatment of venous thromboembolism, a type of cardiovascular disease. The drug has reached Phase 3 trials and received its first approval in the United States in June 2017. Its Fast Track designation highlights the need for new treatments in this therapeutic area.