Analysis on the Clinical Research Progress of Nuclear Factor
NF-κB is present in almost all animal cells and is a key transcription factor in various biological processes in the human body. It participates in cell responses to stimuli such as stress, cytokines, free radicals, ultraviolet rays, and antigens. It plays a central role in cardiovascular development and homeostasis, stress responses, and inflammatory reactions. It also participates in processes related to synaptic plasticity and memory. The most classic biological process studied in NF-κB is immunity, tumor occurrence, and development.
Disorders in the regulation of NF-κB are associated with cancer, inflammation and autoimmune diseases, viral infections, septic shock, and abnormal development of the immune system.
Nuclear Factor Competitive Landscape
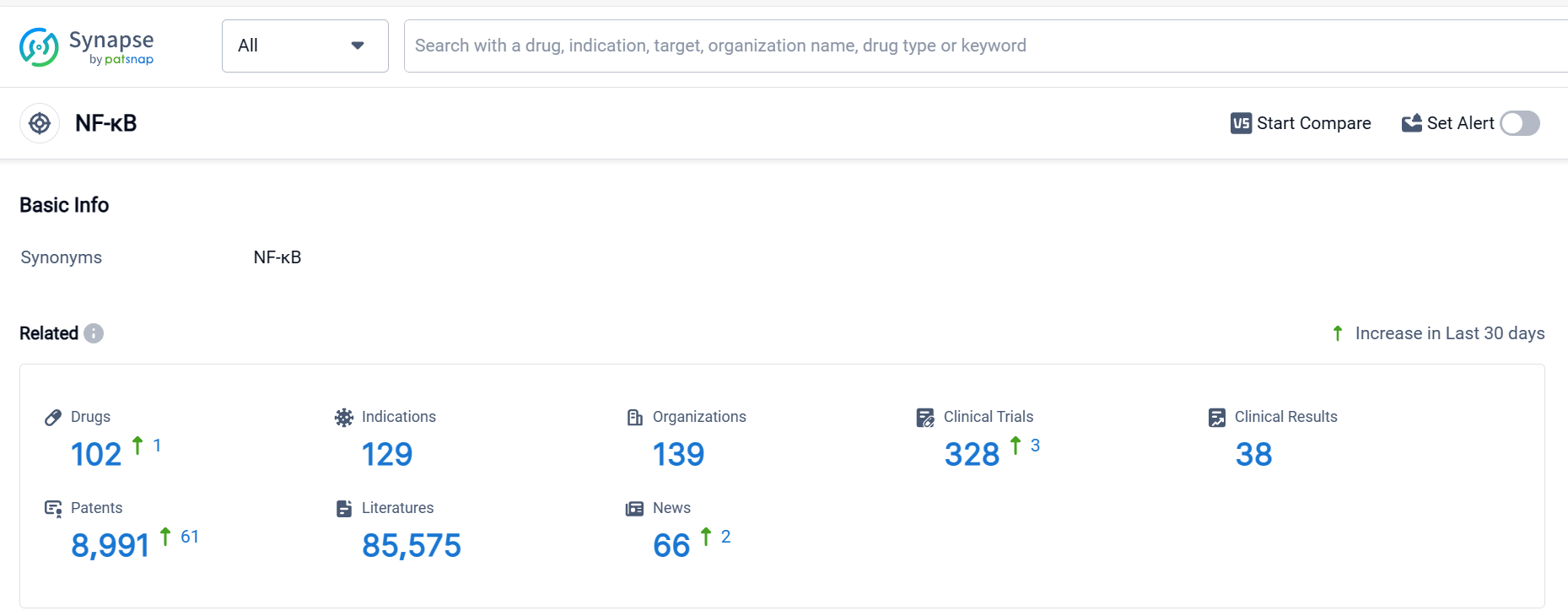
According to Patsnap Synapse, as of 6 Oct 2023, there are a total of 102 NF-κB drugs worldwide, from 139 organizations, covering 129 indications, and conducting 328 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The target NF-κB in the pharmaceutical industry has attracted the attention of several companies, with Especialidades Farmacéuticas Centrum SA, Bayer AG, Mitsubishi Chemical Group Corp., Simcere Pharmaceutical Group Limited, Taisho Pharmaceutical Holdings Co., Ltd., FUJIFILM Holdings Corp., Eisai Co., Ltd., Viatris Inc., Reata Pharmaceuticals, Inc., Kirin Holdings Co., Ltd., Astria Therapeutics, Inc., Mallinckrodt Plc, Hutchison MediPharma Ltd., AnGes, Inc., Osaka University, Nippon Zoki Pharmaceutical Co. Ltd., Loma Linda University, Encore Pharmaceuticals, Inc., Lundbeck Foundation, and Shionogi & Co., Ltd. being the fastest-growing companies.
Drugs under the target NF-κB have been approved for various indications, including Rheumatoid Arthritis, Dermatitis, Atopic, Psoriasis, Osteoarthritis, Chronic Kidney Diseases, Systemic Lupus Erythematosus, Arthritis, Pruritus, Leishmaniasis, Spondylosis, Ankylosing Spondylitis, Malaria, Giardiasis, Periarthritis, Nephritis, Hereditary, Colitis, Ulcerative, Alzheimer Disease, Hepatic Encephalopathy, Muscular Dystrophy, Duchenne, and Diabetic Nephropathies.
Small molecule drugs are progressing most rapidly under the target NF-κB, indicating intense competition around the innovative drug. However, the research and development institutions of biosimilars are not specifically mentioned in the provided data.
China, Japan, and Taiwan Province are developing rapidly under the target NF-κB, with significant progress in drug development. Other countries/locations, such as the United States, European Union, United Kingdom, Canada, Germany, Australia, Israel, France, Spain, Netherlands, Italy, Belgium, Sweden, Czechia, Puerto Rico, Mexico, and Argentina, are also making progress in drug development.
Overall, the current competitive landscape of the target NF-κB in the pharmaceutical industry is dynamic, with multiple companies, indications, drug types, and countries/locations involved. The future development of this target holds promise for the treatment of various diseases and conditions.
Key drug:Iguratimod
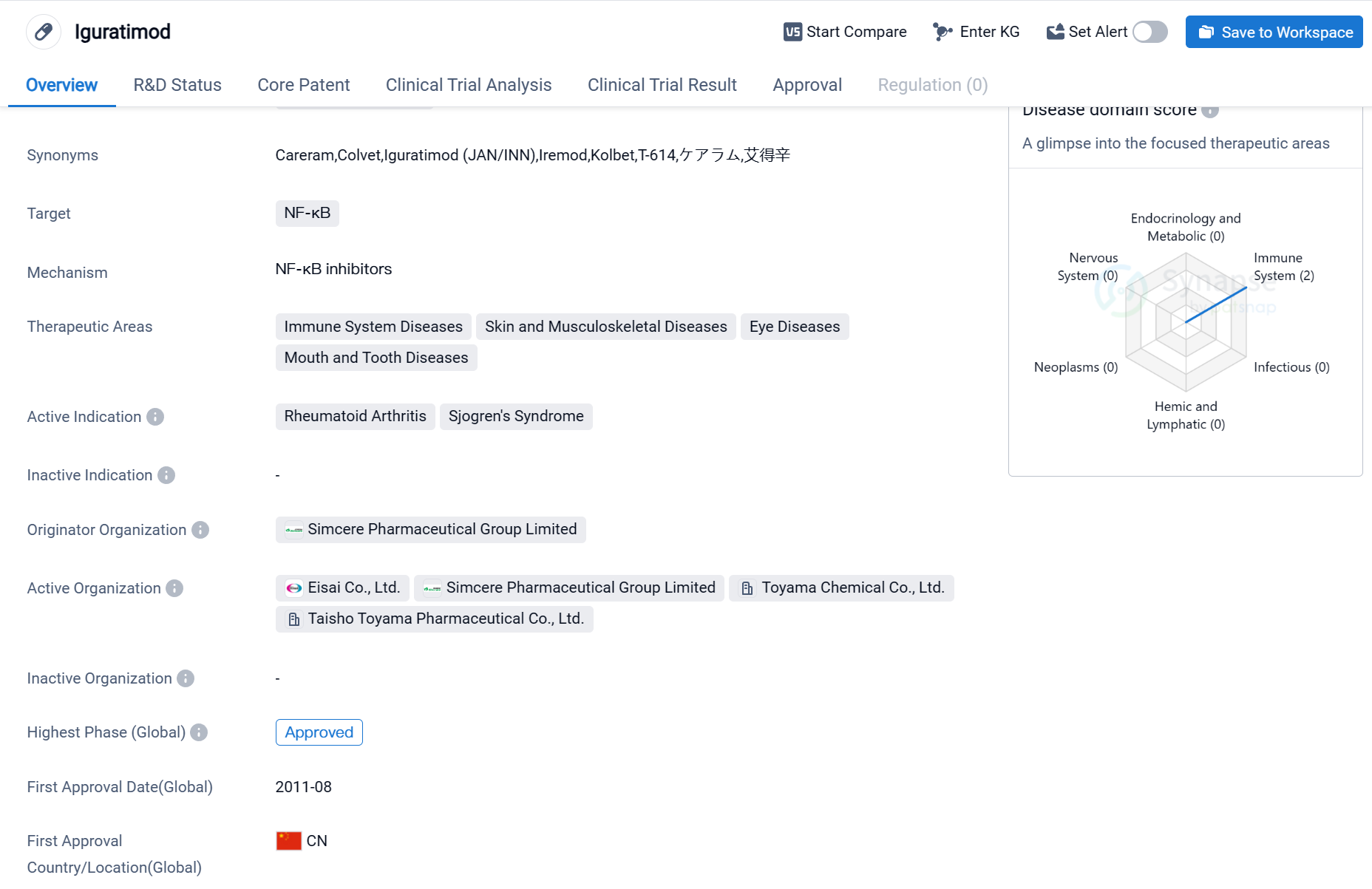
Iguratimod is a small molecule drug that targets NF-κB, a protein complex involved in regulating the immune response. It is primarily used in the treatment of immune system diseases, skin and musculoskeletal diseases, eye diseases, and mouth and tooth diseases. The drug has been approved for the treatment of rheumatoid arthritis and Sjogren's syndrome.
Iguratimod was developed by Simcere Pharmaceutical Group Limited, a pharmaceutical organization based in China. It has received approval for use in the global markets. The drug was first approved in China in August 2011.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Iguratimod is designed to interact with specific molecular targets in the body, in this case, NF-κB. By targeting this protein complex, Iguratimod aims to modulate the immune response and alleviate symptoms associated with immune system disorders.
Rheumatoid arthritis is a chronic autoimmune disease that primarily affects the joints, causing pain, inflammation, and stiffness. Sjogren's syndrome is another autoimmune disorder that primarily affects the moisture-producing glands, leading to dry eyes and mouth. Iguratimod has been shown to be effective in managing the symptoms of both conditions.
The approval of Iguratimod in China in 2011 marked an important milestone in the treatment of rheumatoid arthritis and Sjogren's syndrome. It provided patients with a new therapeutic option to manage their symptoms and improve their quality of life.
In summary, Iguratimod is a small molecule drug developed by Simcere Pharmaceutical Group Limited. It targets NF-κB and is approved for the treatment of rheumatoid arthritis and Sjogren's syndrome. Its approval in China in 2011 has provided patients with a valuable treatment option for these conditions.